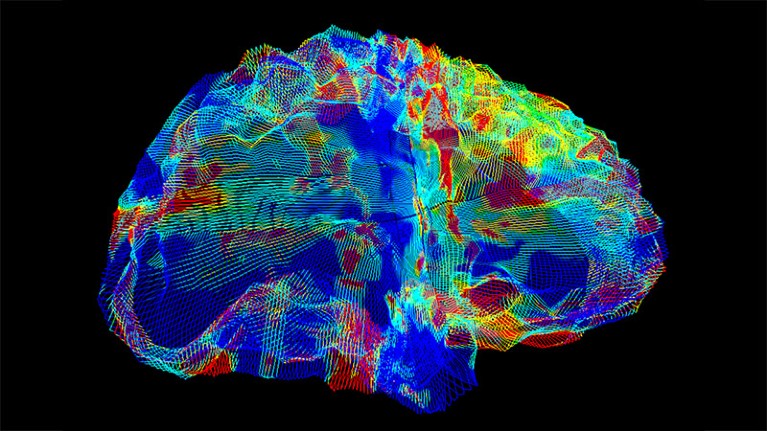
A type of MRI scan (artificially coloured) shows the brain of a person with Alzheimer’s disease.Credit: Mark and Mary Stevens Neuroimaging and Informatics Institute/Science Photo Library
Washington DC
Researchers have sifted through genomes from thousands of individuals in an effort to identify genes linked to Alzheimer’s disease. But these scientists have faced a serious obstacle: it’s hard to know for certain which of those people have Alzheimer’s. There’s no foolproof blood test for the disease, and dementia, a key symptom of Alzheimer’s, is also caused by other disorders. Early-stage Alzheimer’s might cause no symptoms at all.
Now, researchers have developed artificial intelligence (AI)-based approaches that could help. One algorithm efficiently sorts through large numbers of brain images and picks out those that include characteristics of Alzheimer’s. A second machine-learning method identifies important structural features of the brain — an effort that could eventually help scientists to spot new signs of Alzheimer’s in brain scans.
Conquering Alzheimer’s: a look at the therapies of the future
The goal is to use people’s brain images as visual ‘biomarkers’ of Alzheimer’s. Applying the method to large databases that also include medical information and genetic data, such as the UK Biobank, could allow scientists to pinpoint genes that contribute to the disease. In turn, this work could aid the creation of treatments and of models that predict who’s at risk of developing the disease.
Combining genomics, brain imaging and AI is allowing researchers to “find brain measures that are tightly linked to a genomic driver”, says Paul Thompson, a neuroscientist at the University of Southern California in Los Angeles, who is spearheading efforts to develop these algorithms.
Thompson and others described the new AI techniques on 4 November at the annual conference of the American Society of Human Genetics in Washington DC.
Overwhelmed with data
Table of Contents
Thousands of people have had both their genomes sequenced and their brains scanned in the past two decades as part of efforts to build massive research databases. But the rate at which this torrent of information is being produced is outpacing researchers’ ability to analyse and interpret it.
“We’re very data-rich these days compared with how things were 5–10 years ago, and that’s where AI [and machine learning] approaches can excel,” says Alison Goate, a geneticist at the Icahn School of Medicine at Mount Sinai in New York City.
In 2020, Thompson launched AI4AD, a consortium of researchers across the United States that aims to develop AI tools to analyse and integrate genetic, imaging and cognitive data relating to Alzheimer’s disease. As part of this project, researchers created an AI model trained on tens of thousands of magnetic resonance imaging (MRI) brain scans. These images had previously been reviewed by physicians, who picked out scans that showed evidence of Alzheimer’s. From the images, the AI tool learned what the brains of people with and without Alzheimer’s look like.
Self-taught algorithm
In one trial, reported in a preprint1 that has not yet been peer reviewed, the AI classifier detected Alzheimer’s in brain scans with an accuracy of more than 90%. The consortium has also used a similar approach to create a classifier that can accurately sort scans into separate categories according to specific pathological changes in the brain that are associated with cognitive decline and dementia2.
Degui Zhi, a data scientist at the University of Texas Health Science Center at Houston, and his colleagues have taken a different approach. Whereas Thompson and his team focused the AI model on areas of the brain that are known to be linked to Alzheimer’s, Zhi wanted the tool to learn for itself the structural features of the brain that can help to diagnose the disease.
How AI could lead to a better understanding of the brain
The researchers’ AI tool reviewed thousands of brain scans and chose the features that most reliably differentiated one person’s brain from another’s3. Zhi says that this minimizes the likelihood of human bias influencing the algorithm. Now, Zhi’s team is using the algorithm to identify the traits that best distinguish between brain scans of people with and without Alzheimer’s.
Thompson and Zhi acknowledge that the AI models are only as good as the data on which they’re trained. There is a lack of racial and geographical diversity in individuals who have had their brains scanned and genomes sequenced, especially in databases such as the UK Biobank, so the findings from this AI-guided research might not be applicable to everyone. Furthermore, Goate says it will be crucial to show that the AI models’ performance can be replicated in other databases, and that they show consistent results.
Rudolph Tanzi, a neurogeneticist at Massachusetts General Hospital in Boston, says that these biomarkers could one day become part of a set of risk scores for the disease that also integrate blood-based biomarkers and genetics. When all of these bits of data are combined, risk scores can become “exponentially more sensitive”, which will hopefully allow people to seek early treatment before the disease progresses, he adds.
Alzheimer’s is just the beginning, Thompson says. If this approach works, it could also be applied to other diseases that have a physical presentation on brain imaging, he says.
