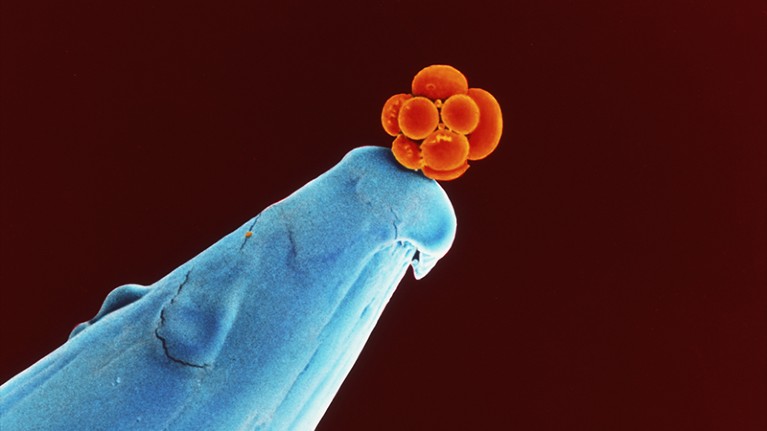
A human embryo at the 16-cell stage sits on the tip of a pin. Researchers say genome-editing techniques are still not safe enough to be used in embryos destined for reproduction.Credit: Dr Yorgos Nikas/Science Photo Library
More than four years after the first children with edited genomes were born, genome-editing techniques are still not safe enough to be used in human embryos that are destined for reproduction, organizers of the Third International Summit on Human Genome Editing announced at the conclusion of the meeting.
“Heritable human genome editing remains unacceptable at this time,” they said in a statement issued on 8 March. “Preclinical evidence for the safety and efficacy of heritable human genome editing has not been established, nor has societal discussion and policy debate been concluded.”
Beyond CRISPR babies: how human genome editing is moving on after scandal
The statement came at the end of a day of discussion and debate in London about the potential of altering the genomes of either embryos or reproductive cells, called gametes, in ways that would be passed down to future generations. Many of the talks at the meeting were focused on technical and scientific challenges, such as the uncertain consequences of breaking both strands of the DNA double helix — a necessary step in some forms of genome editing — in embryos.
In addition to those challenges, society must grapple with questions about whether the technology should be deployed, organisers said: “Governance frameworks and ethical principles for the responsible use of heritable human genome editing are not in place.”
Effects of editing
Table of Contents
Some researchers have argued that heritable genome editing could help people who carry genetic diseases to avoid passing those conditions on to their children. In many cases, this can already be done by combining in vitro fertilization (IVF) with testing of the resulting embryos for a given genetic disorder. But that is not always an option, such as when all a couple’s embryos will inevitably inherit the genetic disorder, or when all available embryos happen to carry the responsible genes.
In addition to broader concerns about ethics and social justice, editing embryos would require a safe and effective genome-editing platform to minimize the chances of harming the embryo, the resulting child, and that child’s descendants. Most research on genome editing in embryos, however, has been done using animal models such as mice, which might not accurately reflect what happens in human embryos. And although potential genome-editing therapies have been widely studied in adult human cells, embryos might respond differently than adult cells to the DNA damage caused by some genome editing tools.
Only a handful of laboratories have directly tried to edit the genomes of human embryos using the popular editing system CRISPR-Cas9, and several of these presented concerning results at the summit.
CRISPR gene editing in human embryos wreaks chromosomal mayhem
The Cas9 enzyme works by breaking both strands of DNA at a site designated by a guiding piece of RNA. The cell then repairs that DNA break, either by using an error-prone mechanism that stitches the two ends together but sometimes deletes or inserts a few DNA letters in the process, or by replacing the missing DNA with a sequence copied from a template provided by the researcher. DNA breaks created by Cas9 in embryos are usually repaired using the error-prone pathway, rather than using the template DNA, said Deitrich Egli, a stem cell biologist at Columbia University in New York City, at the conference.
Egli and other researchers also reported on the consequences of the double-strand breaks made by Cas9. Developmental biologist Kathy Niakan at the University of Cambridge, UK, recounted her lab’s experience with the apparent loss of large regions of chromosomes that occurred when using CRISPR-Cas9 to edit human embryos1. Shoukhrat Mitalipov, a reproductive biologist at Oregon Health & Science University in Portland, also said that his laboratory had found large DNA deletions at the editing site in human embryos, and that these deletions might not be detected using standard tests2.
“Can human embryos at this stage really tolerate this kind of intervention?” asked Dagan Wells, a reproductive geneticist at the University of Oxford, UK, who also reported concerning responses to DNA breaks in human embryos. About 40% of the embryos in one of his genome-editing studies failed to repair broken DNA. Over one-third of those embryos continued to develop, he said, resulting in the loss or gain of pieces of chromosomes in some cells. That could risk the health of offspring if such embryos were allowed to develop further. “These results are really a warning,” he said.
Better techniques
There are newer variations on CRISPR-Cas9 editing that do not break both strands of the DNA helix. Base editing, for example, can convert one single DNA letter to another, and a technique called prime editing allows researchers to insert DNA sequences more predictably than CRISPR-Cas9 editing. Neither of these methods cause double-strand breaks, but they have not been as thoroughly studied and optimized as CRISPR-Cas9. At the summit, developmental biologist Yuyu Niu at the Kunming University of Science and Technology in China reported that one kind of base editor did not cause off-target DNA mutations in rhesus macaque (Macaca mulatta) embryos, but it did cause unwanted RNA mutations3.
Super-precise CRISPR tool enhanced by enzyme engineering
An alternative to editing embryos would be to instead edit gametes, such as eggs and sperm, or the stem cells that give rise to them. This would also sidestep concerns that efforts to edit embryos might not succeed in all cells of the embryo, resulting in offspring that contain a mixture of edited and unedited cells. Several researchers at the summit reported progress towards generating gametes in the laboratory, but doing this with human cells destined for reproductive uses still poses challenges.
The summit organizers urged that researchers continue to explore each of these options, even as policy makers and the public grapple with what restrictions should be placed on heritable genome editing.. “We are still keen that the research goes ahead,” said developmental biologist Robin Lovell-Badge of the Francis Crick Institute in London, who chaired the organizing committee for the summit. “In parallel, there has to be more debate about whether the technique is ever used.”
