Since 2022, member states of the World Health Organization (WHO) have been negotiating a new treaty — provisionally termed the Pandemic Agreement. If adopted, it would transform how the world handles pandemic prevention, preparedness and response. Opinions differ on what negotiators should prioritize. But no issue has captivated public attention as much as vaccine equity — or done more to bring countries to the negotiating table.
During the COVID-19 pandemic, scientists began to design vaccine candidates only a few hours after the first SARS-CoV-2 genome sequence was shared. By the end of 2020, mass vaccination had begun in the United States and Europe. High-income countries promised to share vaccines through the voluntary WHO COVID-19 Vaccines Global Access (COVAX) programme, but failed to meet their commitments. When South Africa and India appealed to the World Trade Organization for an emergency waiver of intellectual-property rights related to COVID-19 vaccines, so that every country could start their own manufacturing, high-income countries blocked the proposal for months. The refusal of wealthier nations to cooperate had cost between 200,000 and 1.3 million lives by the end of 2021 in low- and middle-income countries1,2. Today, nearly one-third of the world’s population has still not received a single dose, and the death toll resulting from vaccine nationalism continues to grow.
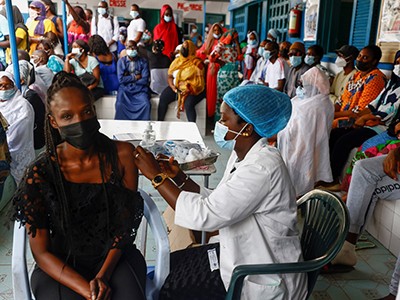
Global vaccination must be swifter
The Pandemic Agreement could be the last chance to fix this problem before the next COVID-19 arrives. Yet the proposed solution — the Pathogen Access and Benefit-Sharing (PABS) System, which was outlined in Article 12 of the latest treaty draft — still hangs in the balance. The second-to-last session of the treaty’s Intergovernmental Negotiating Body is now under way. So far, countries have been unable to agree on this part of the text. As time runs out, we urge WHO member states to agree on a ‘science-for-science’ mechanism that ensures vaccine equity in the next pandemic.
The road to PABS
Table of Contents
Across all fields, scientists from the global north have frequently extracted data and samples from the global south without the permission of the people there, without collaborating meaningfully — if at all — with local scientists, and without providing any benefit to the countries where they conduct their work. In 1993, the Convention on Biological Diversity recognized parties’ sovereign rights to their ‘genetic resources’. Since 2014, under the Nagoya Protocol on Access and Benefit-sharing, countries have developed their own legislation to ensure that they receive benefits (such as financial compensation or scientific collaboration) when scientists and others from outside the country access their genetic resources.
Discussions on access and benefit-sharing in global health began in earnest in 2007, when the Indonesian government refused to share avian influenza samples with the rest of the world, on the grounds that such samples were often used to make vaccines that were never made available in most places3. Sparked by this conflict — and the 2009 H1N1 flu pandemic — WHO member states developed the 2011 Pandemic Influenza Preparedness (PIP) Framework to streamline the sharing of influenza viruses with pandemic potential, as well as vaccines and other benefits.
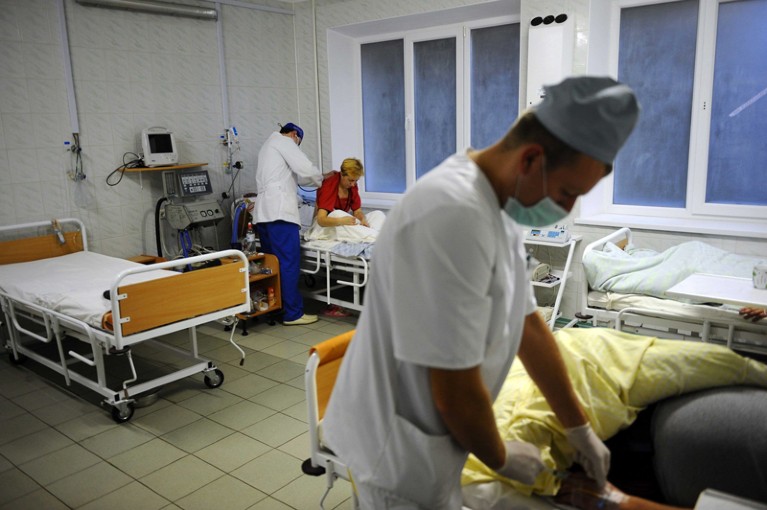
After the 2009 H1N1 influenza pandemic, the World Health Organization designed a plan for global sharing of flu viruses and vaccines. The same must be done for all viruses with pandemic potential.Credit: James Hill/Redux/eyevine
Under the PIP Framework, 14 manufacturers have promised that when the next influenza pandemic starts, they will share up to 10% of the vaccines that they make (around 420 million doses) with the WHO. In exchange, these companies have access to a global network of laboratories and their flu samples. The PIP model shows significant promise, but is so far untested and applies only to influenza.
The proposed PABS System in the Pandemic Agreement would take lessons from the PIP Framework and apply an access and benefit-sharing scheme to any pathogen with pandemic potential, such as SARS-CoV-2. Under the PABS System, scientists would share pathogen samples and data through a global network of laboratories and sequence data repositories. In exchange for access to samples and data, manufacturers of vaccines or therapeutics would give at least 20% of their products to the WHO (half for free, and half at affordable prices). The WHO would then distribute these on the basis of public-health risk and needs. Users of the PABS System would also contribute to a capacity-development fund, and be encouraged to explore other kinds of benefit-sharing, such as scientific collaborations and technology transfer.
Science-for-science
With regard to physical samples, the Nagoya Protocol and its national implementing legislation can be cumbersome to navigate4. Some scientists are apprehensive about the idea of introducing similar barriers into work with genetic sequence data, especially during outbreaks.

Global ‘pandemic treaty’: nations wrestle with how to fairly share virus data
In relation to the Nagoya Protocol, several professional societies, including the American Society for Microbiology, have endorsed a group of US scientists that opposes “any restriction or control of access and/or use” of any genetic sequences (see go.nature.com/3i5ds). Comments from sessions indicate that such concerns are increasingly being echoed by representatives of global north countries in the current Pandemic Agreement negotiations. Some critics have even argued that the proposals for PABS would block progress towards open science, in favour of a transactional approach5.
As a collective of 290 scientists from 36 countries, we argue that a pandemic treaty cannot succeed unless it ensures that everyone will benefit from pandemic science.
Under the new treaty, should it be adopted with the current vision of the PABS System, countries will still be expected to ensure that their scientists share lifesaving data openly and rapidly. Scientists will still be able to share their data freely outside of PABS platforms, and widely used databases could enter into the PABS System — meaning that most researchers would never experience any disruptions to their workflow. The WHO could also establish its own repository or clearinghouse for genetic sequence data and samples, which would potentially provide scientists with more transparent management of these resources and the guarantee of continued access.
Financing committed largely by pharmaceutical firms using these platforms (which sometimes directly derive profits from publicly funded science) would, in turn, go towards expanding sequencing capacity and scientific research in low-resource settings. It would also help to support other priorities, such as pandemic prevention6. What’s more, scientists everywhere, but especially in the global south, would benefit from a system that creates opportunities for international collaboration — and that ensures that people receive credit for sharing their data.
Hold the course
Access and benefit-sharing could just as easily be called ‘science for science’: the PABS System will support more pandemic science, and ensure that scientists’ contributions result in their communities having access to lifesaving advancements.
Earlier this week, the Intergovernmental Negotiating Body for the Pandemic Agreement reconvened for its penultimate session. If Article 12 is weakened or dismantled, it will be a monumental setback for global health justice — and for the global scientific community.
Although today’s scientific community has embraced the ideals of open data sharing, the world is no closer to a fair system for sharing vaccines and therapeutics. Intellectual property, not benefit-sharing, is the antithesis of open science. We dream of a world in which such barriers are dismantled for lifesaving medicines. Until that day, the Pandemic Agreement offers the last best chance to avoid repeating the mistakes made during the COVID-19 pandemic.
Competing Interests
C.C. has previously received funding support from the Coalition for Epidemic Preparedness Innovations, and has been a consultant for the US Department of State on Global Health issues. D.B. is a member of the Lancet-PPATS Commission on Prevention of Viral Spillover. A.P. has advised multiple countries pro bono on the treaty negotiations and has consulted for the WHO on international law. C.C. and A.P. receive funding support from the Carnegie Corporation of New York and the US National Science Foundation for research related to the Pandemic Agreement. We declare no other competing interests.
