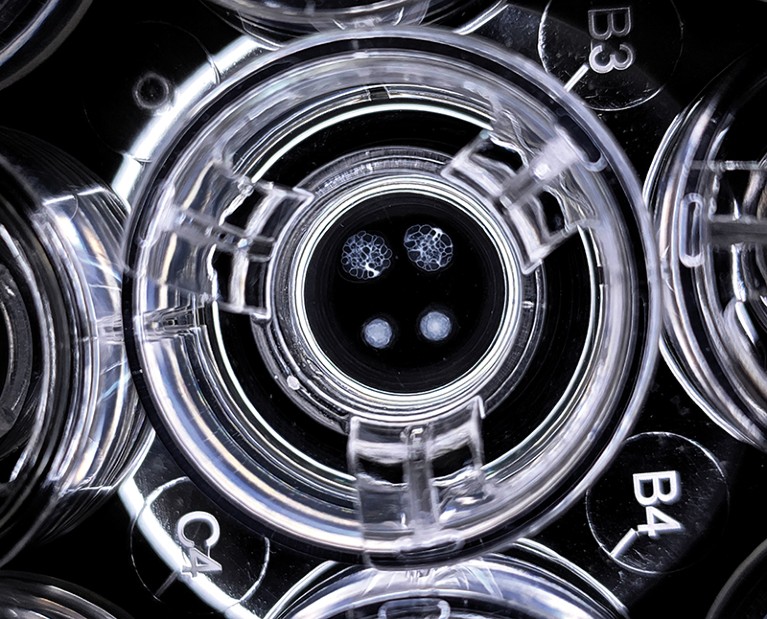
Kidney-like structures called organoids can be grown from stem cells.Credit: Xia Lab
Ryuichi Nishinakamura’s quest to build a transplantable kidney began in the 1990s, when the nephrologist found he had little to offer his patients. At times he was ridiculed for setting such an unrealistic goal, “but I was very naive and young, so I just went forward”, recalls Nishinakamura, who is now a stem-cell biologist at Kumamoto University in Japan.
But the discoveries of human embryonic stem cells in 1998 and of a way to create induced pluripotent stem (iPS) cells in 2006 made the task of growing fresh kidneys seem more achievable. Many investigators differentiated various types of kidney cell from iPS cells and grew kidney ‘organoids’ — tiny, organ-like structures with multiple types of kidney cell that partly mimic kidney structure and function. In 2013, Nishinakamura’s lab achieved one of the early milestones, demonstrating both mouse and human kidney organoids1. Many labs are now producing ever-more functional organoids that are proving useful in modelling kidney development and disease.
Part of Nature Outlook: Chronic kidney disease
But Nishinakamura’s goal of a transplantable human kidney is still many years away. “We do have kidney in a dish,” says Melissa Little, a developmental biologist at the Murdoch Children’s Research Institute in Melbourne, Australia. “But will it be useful if we transplant it? That’s a much bigger question.”
“We are at a bottleneck,” says Yun Xia, a stem-cell biologist at Nanyang Technological University in Singapore, who sees an enormous gap between today’s research and what people need. Like many of her colleagues, Xia worries that to keep its credibility with funders and the public, organoid research needs to show progress towards treatments. Some groups are working towards ‘auxiliary kidneys’, which would be a fraction of the size of a normal kidney but could still stabilize a person’s health.
Challenges of complexity
Table of Contents
The kidney is an exceptionally tricky organ to replicate in a lab. “You’ve got 25–30 distinct cell types with functional roles that have to be anatomically placed in the right position for the organ to work,” says Little. By contrast, the heart is thought to have only nine major cell types2.
The kidney’s functional unit for removing waste from the blood, the nephron, is an intricate and precisely organized structure. The first step in filtering the blood takes place in networks of small blood vessels called glomeruli. The resulting filtrate then passes through a series of tubes, in which various solutes are exchanged with blood vessels, before ending up in a branching tree of collecting ducts that funnels the waste to the ureter and out towards the bladder. For a kidney to function, it is not enough to simply have the right cells — they must also be arranged correctly.
A number of daunting obstacles remain on the road to a transplantable kidney. One of the biggest is immaturity of the cells, which typically resemble progenitor cells from the first or second trimester of human development, limiting their functionality.
There has been steady progress on this front. In 2022, for instance, Little and her colleagues demonstrated more functional human proximal tubule cells, which she calls the “powerhouses” of the kidney3. The lab of nephrologist Joseph Bonventre at Brigham and Women’s Hospital in Boston, Massachusetts, did the same that year for the collecting duct’s two main functional cell types4.
Researchers have also worked out how to boost their ability to create kidney organoids in volume, another key requirement for potential treatments. For example, researchers in the Netherlands have grown sheets of iPS-cell-derived nephrons at a large scale5.
Like other forms of tissue derived from pluripotent stem cells, kidney organoids can include undesired off-target cells such as muscle neurons, and researchers need to follow precise protocols to guard against the appearance of tumour cells. General advances in the stem-cell field are minimizing these challenges.
Forming a vasculature, however, is a much greater hurdle. A fully developed and precisely structured blood system is needed to keep the flows of blood and urine exchanging correctly throughout the nephron. This has not been achieved in experimental systems, says Jamie Davies, a developmental biologist and tissue engineer at the University of Edinburgh, UK. Instead, the vasculature generally remains in a primitive state and soon dies out.
Organoids transplanted into immunodeficient mice do attract blood vessels from the host animal, allowing nephrons to start filtering the blood and generating urine, says Nishinakamura. However, the urine has nowhere to go, so transplants typically fail at that stage, he says.
Adding dimension to modelling
The push to build better organoids based on iPS cells has vastly increased researcher’s understanding of kidney development and disease. Compared with cell cultures, organoids already offer enhanced models of kidney disease — particularly for genetic illnesses in children. For example, the crucial cells that wrap around glomerulus capillaries and begin the filtering process, called podocytes, are “rubbish” in 2D cell cultures but much better representations in 3D organoids, Little says.
Organoid models also readily display the characteristic cysts of autosomal dominant polycystic kidney disease — the most prevalent genetic kidney disease and one subject to intense research. One 2022 study reported a scalable human kidney organoid platform that enabled the testing of hundreds of small-molecule drugs against this condition6.
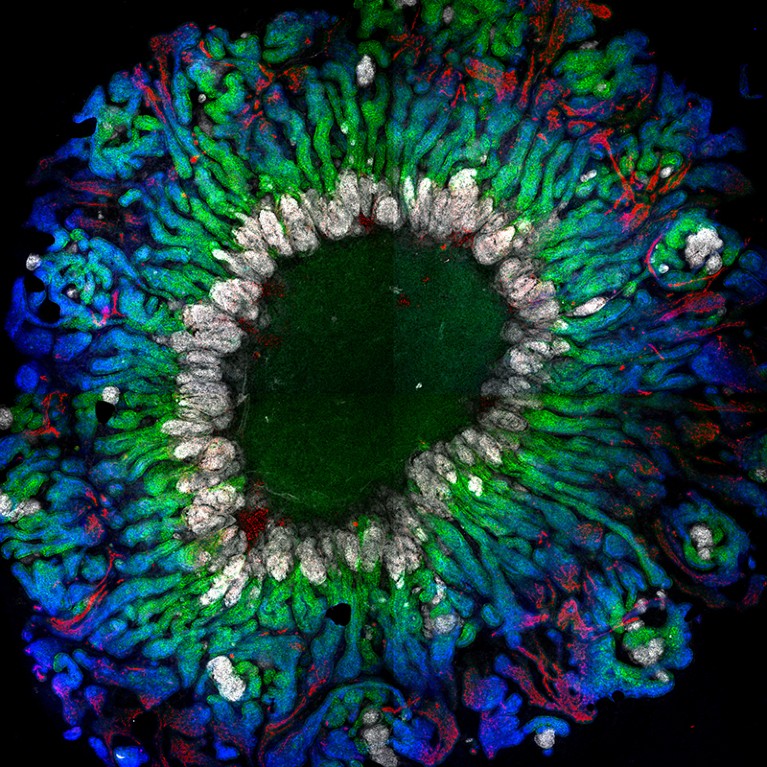
Researchers are now able to grow kidney organoids with more-functional cells.Credit: J. M. Vanslambrouck et al. Nature Commun. 13, 5943 (2022).
Diabetes is the largest driver of chronic disease in adults but a formidable task to model, because the condition impairs the blood vessels that are difficult to reproduce in organoids. Moreover, says Xia, kidney organoids, like many cell cultures, are generally bathed in high levels of glucose, making it hard to pick out the effects of the raised blood glucose levels generated in diabetes.
Kidney organoids show great promise in drug testing. Many drug candidates fail testing because they cause kidney damage, but this is not picked up in 2D cell cultures because they often lose their sensitivity, says Bonventre. For example, he says, the protein KIM-1 is a strong biomarker for damage to proximal tubule cells in vivo — but not in 2D cell culture. If kidney organoids can display the same KIM-1 gene-expression patterns that are seen in vivo, they will provide excellent toxicity models, he says. His lab is studying such organoid-based models.
Tangible steps towards treatment
Using organoids based on iPS cells as a treatment for kidney disease is far from the first cell-based therapy to be proposed. Many clinical trials have tested the effect of mesenchymal stem cells (multipotent stem cells found in tissue, such as bone marrow), with mixed results. Most researchers agree that although these cells might secrete factors that help with kidney repair, they don’t structurally improve the kidney. One long-studied alternative technique that selects, enhances and reinserts kidney cells from people with kidney disease is being examined in a phase III clinical trial sponsored by the biotechnology company ProKidney in Winston-Salem, North Carolina.
But injecting iPS-cell-based organoid-derived cells alone into kidneys doesn’t seem to be a promising strategy, says Nishinakamura. Such cells might secrete factors that improve kidney function, much as mesenchymal stem cells are thought to do, he says. But these progenitor cells are unlikely to stay and play happily within the kidney; it’s not clear where the cells might go, or if and how they then mature.
Organoid-derived cells might help when it comes to improving transplants of donated kidneys, says Núria Montserrat, a stem-cell biologist at the Institute for Bioengineering of Catalonia in Barcelona, Spain. She is testing that hypothesis in collaboration with Cyril Moers, a transplant surgeon at the University of Groningen in the Netherlands. Donated organs are often maintained before transplant by being perfused in a liquid bath rather than frozen. Moers hopes that adding organoid-derived cells to these baths will allow these organs to be preserved better, evaluated more accurately and (eventually) made healthier before transplant. Montserrat’s lab is running pilot experiments with human organoid cells released into perfused pig kidneys.
More broadly, a number of groups are studying the potential for transplanting a more substantial set of organoid tissues into people with kidney disease. Little wants to create what she calls an auxiliary kidney, designed to connect to a person’s failing kidney.
In her lab’s unpublished experiments, human kidney organoids transplanted into immunocompromised mice successfully gather blood vessels and start filtering urine. “Getting all those nephrons to connect to the underlying kidney will be the challenge,” she says. “If the nephrons connect into the existing kidney itself, then the urine will go out the way all of the urine goes. You’re essentially freeloading on the anatomy of the existing patient kidney, even though that patient’s kidney is pretty sick.”

More from Nature Outlooks
Biotechnology company Trestle Biotherapeutics in San Diego, California, is also developing a transplantable auxiliary kidney. Co-founder and developmental biologist Alice Chen says that this tissue might end up in another location, such as below the existing kidney near the bladder. Trestle is growing organoids about 100 times the size of those commonly reported in the scientific literature, she says, and is seeing encouraging progress in how these tissues engraft in mice, connect to the host circulation and continue to mature.
The start-up was launched with the view that a bioengineered kidney will demand industrial-scale expertise in many fast-evolving disciplines, including stem-cell science and 3D bioprinting. “We had to pull all of that brainpower, those technologies and those ideas together,” says Chen.
Most people with kidney disease are hoping for treatments that let them live their lives — replacing or minimizing dialysis, or postponing the immediate need for a donated kidney — rather than for a complete bioengineered organ. “We’re not creating an entire organ, we need to create some sort of tissue that can return 10–20% function to these patients,” Chen says. “And that is achievable.”
Some research groups are using organoids as potential sources of cells for hybrid external devices with bioengineered 3D scaffolds, designed to act like improved dialysis systems.
In one such effort, Bonventre hopes to make a device with a sub-population of just two types of cell: proximal tubule cells and collecting duct cells. Other types of kidney cells remain important, he says, but achieving every function of a normal kidney seems like a distant goal. “Let’s not shoot for a galaxy that’s three billion light years away,” he says. “Let’s try to get to the Moon first, and maybe Mars.”
But Nishinakamura remains fixed on his original dream of a complete kidney, which he thinks is needed more than ever for the millions of people whose chronic kidney disease steadily progresses towards end-stage renal disease. “I’m always telling my graduate students, ‘Don’t say it is impossible’.”
