In the past few decades, innovative strategies have been developed to stabilize chemical compounds that contain main-group elements in unusually low oxidation states. (Main-group elements are those on the left and right sides of the periodic table, surrounding the transition metals.) Many of these compounds were previously thought to be impossible to obtain as stable materials. This is especially the case for magnesium, a reactive metal whose compounds almost all contain the element in the +2 oxidation state (equivalent to the atoms losing two electrons and having a charge of +2). Writing in Nature, Rösch et al.1 report that magnesium can form compounds in which it keeps all of its electrons, and thereby exists in the zero oxidation state that is normally characteristic of the pure element. These magnesium(0) compounds are stable at room temperature, but are highly reactive, with the metal atoms readily surrendering electrons in chemical reactions that are potentially useful for organic synthesis.
It is well established that transition metals can exist in a range of oxidation states in their compounds, including the zero oxidation state. This characteristic is central to the ubiquitous application of such compounds as industrial catalysts. By contrast, the highly reactive s-block metals (groups 1 and 2 of the periodic table, excluding hydrogen) were not thought to be capable of forming similar, stable low-oxidation-state compounds, because they tend to lose all of their outermost electrons during compound formation.
This idea was overturned in 2007, when the first compounds of magnesium — a group 2 metal — were prepared in the +1 oxidation state2. These compounds consist of two magnesium atoms connected by a chemical bond, with each magnesium atom bound to a bulky organic ligand; the ligand acts as a protective coating that prevents the magnesium ions from reverting to the +2 oxidation state. Other examples of compounds containing group-2 metals in low oxidation states have since been reported, such as beryllium in the zero3 and +1 oxidation states4, and calcium in the +1 state5, but they typically require special electron-accepting ligands to stabilize the compound concerned.
Such advances in the chemistry of s-block metals have inspired chemists to pursue one of the outstanding goals in the field: the preparation of stable magnesium(0) compounds6. The logical approach would be to react an extremely bulky magnesium(ii) precursor compound with a powerful reducing agent, such as sodium or potassium metal. The idea is that the reducing agent would deliver two electrons to the magnesium atom, thus forming a magnesium(0) compound.
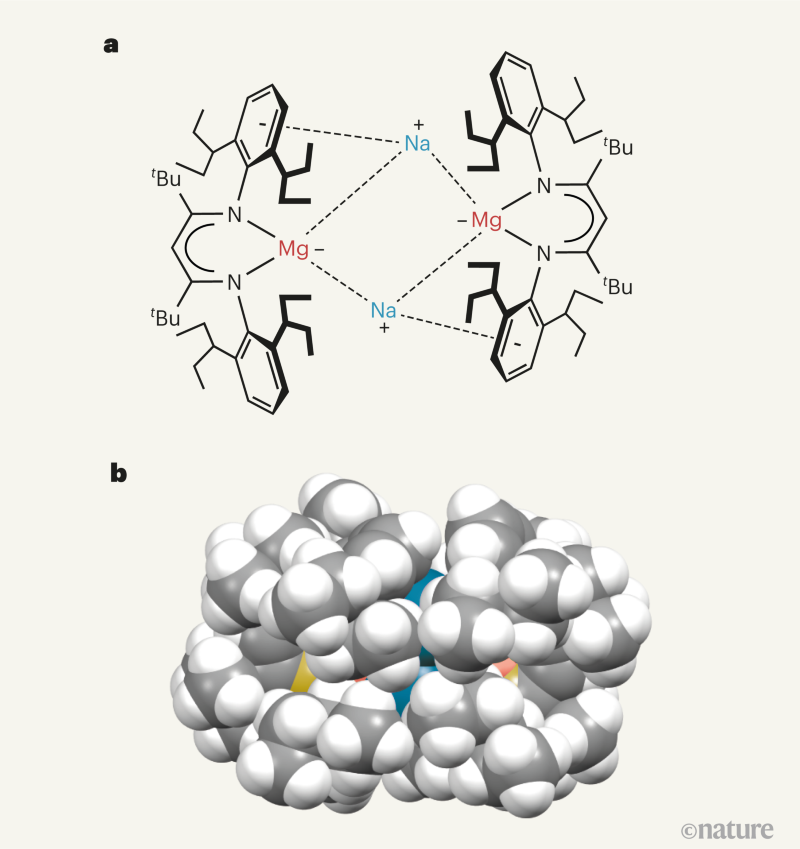
In pursuit of this goal, researchers from the same laboratory as Rösch et al. previously developed an extraordinarily bulky ligand (abbreviated as BDI*), which they used to prepare a magnesium(ii) precursor compound7. However, when they reacted the precursor with potassium metal, only one electron was donated to the magnesium atoms, resulting in the formation of the magnesium(i) compound (BDI*)Mg–Mg(BDI*). Rösch and colleagues now report that, when they change the reducing reagent to a finely divided (powdered) form of sodium metal, two electrons are donated to the precursor, yielding the remarkable magnesium(0) compound {[(BDI*)Mg–][Na+]}2 (Fig. 1).
It is clear that the enormous size of the BDI* ligand is required to stabilize the magnesium(0) compound, preventing the solid form of the compound from decomposing at room temperature. Even so, the compound partially decomposes at room temperature when in solution. This process yields another unprecedented type of compound known as a magnesium cluster, in which three magnesium atoms are bonded together, (BDI*)Mg–Mg–Mg(BDI*). Astonishingly, this contains magnesium in both the 0 and +1 oxidation states. Rösch et al. reasonably posit that the formation of this compound could shed light on the unknown mechanism of formation of Grignard reagents — an important class of magnesium(ii) compound that has been widely used in organic chemistry for more than 120 years.
The size and 3D shape of the BDI* ligand hits the sweet spot when it comes to stabilizing the magnesium(0) compound. The authors report that the compound consists of a central core of magnesium and sodium atoms — [Mg2Na2]2+ — arranged in a ring and enveloped by two BDI* ligands (Fig. 1). Computational analyses reveal that the magnesium atoms have the same number of electrons as magnesium metal, which means that the compound could be viewed as a soluble form of the metal. There is, however, some sharing of electrons between the magnesium and sodium atoms. This doesn’t detract from the assignment of the oxidation state of the magnesium atoms as zero, and the observation of a magnesium–sodium ‘bond’ in the compound is itself another first.
Given that the magnesium atoms are in the zero oxidation state, the compound should display a level of reactivity similar to that of the elemental metal. In fact, Rösch and co-workers’ preliminary experiments show that it is even more reactive than that. For example, it can readily activate (break or weaken) very strong bonds, such as hydrogen–hydrogen and carbon–fluorine bonds, at room temperature. Many other compounds that contain main-group elements in low oxidation states can do the same8. A true demonstration of the exceptional reducing ability of the magnesium(0) compound would be the activation of even more staunchly inert molecules, such as dinitrogen (N2). This seems achievable, given the recent demonstration that dinitrogen can be activated by a transiently formed calcium(i) compound9.
A more surprising aspect of the reactivity of Rösch and colleagues’ compound is that its magnesium(0) atoms can transfer electrons to its sodium atoms, reducing them back to sodium metal. This seems counter-intuitive, because the reverse process — the reduction of magnesium(ii) to magnesium(0) by sodium metal — was used to make the magnesium(0) compound in the first place. The authors’ experimental evidence backs up the observation that the sodium atoms are reduced, but more work is required to examine the processes by which this operates.
Rösch and co-workers’ stable magnesium(0) compound is a landmark in the chemistry of the s-block elements. It will fundamentally change chemists’ views about what can be synthesized using these elements. Moreover, it will help to advance our understanding of — and raise questions about — the unusual ‘non-classical’ bonding in low-oxidation-state main-group compounds. The development of highly reducing magnesium(0) compounds might also pave the way to their use in chemical reactions that, at present, cannot normally be carried out with s-block metals. The future is surely bright for magnesium now that it has hit zero.