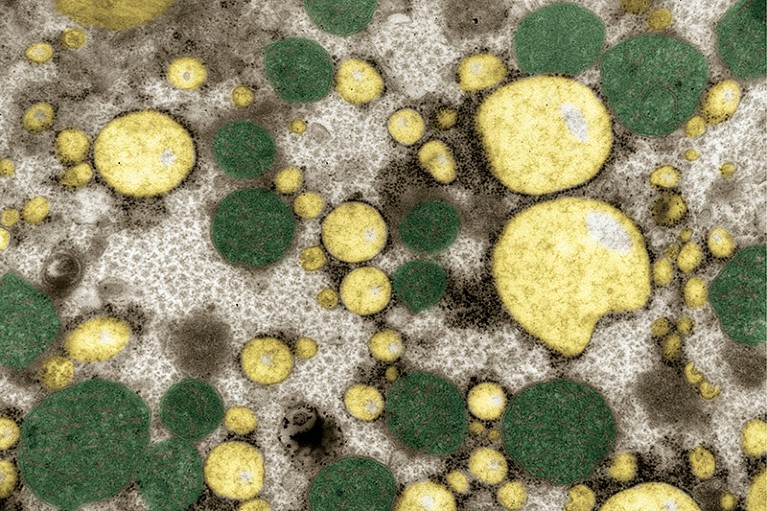
Liver tissue from a person with extra fat in the organ.Credit: IKELOS GmbH/Dr Christopher B. Jackson/Science Photo Library
For the first time, the US Food and Drug Administration has approved a drug to treat an obesity-linked liver disease that is on the rise around the globe and is becoming a leading driver of liver failure and transplants.
The drug, called resmetirom, has been shown to reduce scar tissue in the liver and other hallmarks of a disease called metabolic dysfunction-associated steatohepatitis (MASH). MASH is often associated with the metabolic turmoil that can accompany obesity and diabetes, and in severe cases can lead to liver failure or cancer.
The disease affects an estimated 5% of the world’s adults. “It’s a huge population,” says Na Li, a hepatologist at Ohio State University Wexner Medical Center in Columbus. “And I think we have made a big step forward to improve their care.”
Worth the wait?
Table of Contents
That step has been a long time coming: despite the clear need, pharmaceutical companies have struggled to develop a successful treatment for MASH. Last year, Intercept Pharmaceuticals in Morristown, New Jersey, abandoned a highly anticipated drug called obeticholic acid, amid concerns from the US Food and Drug Administration (FDA) that its modest effectiveness was not enough to outweigh safety risks.
“Many trials over the years have failed, even those that initially looked promising,” says Li. “That’s the tragedy we’ve had.”
Fatty liver disease: turning the tide
MASH is caused by an accumulation of toxic, fatty molecules in the liver. Over time, this leads to inflammation and tissue damage. As the liver begins to accumulate scar tissue, a process called fibrosis, its ability to function declines. (MASH was called nonalcoholic steatohepatitis, or NASH, until professional societies adopted new nomenclature last year.)
Resmetirom boosts the liver’s ability to respond to thyroid hormone, which in turn stimulates the organ’s metabolism of fatty acids. In a year-long, multinational clinical trial in 966 people with MASH, researchers found that the drug reduced inflammation and fat build-up in 30% of participants who received the highest dose of resmetirom, compared with about 10% of those who took a placebo1. Fibrosis improved in about 26% of the highest-dose group, compared with 14% of the placebo group, making resmetirom the first candidate MASH drug to reduce fibrosis. It will be marketed as Rezdiffra and will be available to people with moderate to severe liver scarring.
Long-term benefits in doubt
The drug’s effectiveness, coupled with relatively mild side effects, was exciting and suggested that there could finally be a way to treat MASH, says Maya Balakrishnan, a gastroenterologist at Baylor College of Medicine in Houston, Texas. But the FDA granted resmetirom accelerated approval: for the drug to stay on the market, its developer, Madrigal Pharmaceuticals in Conshohocken, Pennsylvania, will eventually need to provide long-term evidence that it produces meaningful benefits.
“Only time will tell,” says Balakrishnan. “In the end, what matters is: does this drug improve survival?”

Disease progression: Divergent paths
In the meantime, researchers are eagerly anticipating results from a study of semaglutide, a popular weight-loss drug, against MASH. Weight loss has been associated with a reduction in MASH severity, but an early clinical trial of semaglutide in participants with MASH yielded mixed results: some hallmarks of the disease improved, but liver fibrosis did not2. Still, researchers hope that semaglutide could help, and that the larger ongoing trial will provide clearer results, says Li.
In the meantime, resmetirom could be the best recourse for people with MASH. But physicians must be clear about the limited data on the drug when they discuss resmetirom with their patients, says Balakrishnan.
Access will be another issue, she says. Many of the people most in need of treatment are members of disadvantaged communities in which obesity and diabetes are prevalent. They often have limited access to health care, and it’s not yet known how much resmetirom will cost. “Who are the patients who are going to be able to access the medication?” says Balakrishnan. “It’s definitely a big concern.”
Potential blockbuster
Access in other countries will have to wait. Madrigal Pharmaceuticals’ clinical trials of resmetirom focused on the United States, says Claudia Oliveira, a pathologist at the University of São Paulo in Brazil. “We did not get the opportunity to see this drug in patients in Latin America,” she says. “But we all have expectations about the drug, because the results of the trial were very, very interesting.”
“Madrigal is a small company and chose to focus on a more limited geographic footprint for our trials,” a spokesperson for the company told Nature.
That decision likely helped speed the company’s first approval, says Norberto Chavez Tapia, a hepatologist at Médica Sur in Mexico City. Soon, he predicts, resmetirom will be investigated in clinical trials around the world.
After that, depending on the drug’s price and effects on transplants and survival, resmetirom could be welcomed in many health-care systems, says Tapia: “It’s a very attractive drug for us, worldwide.”
