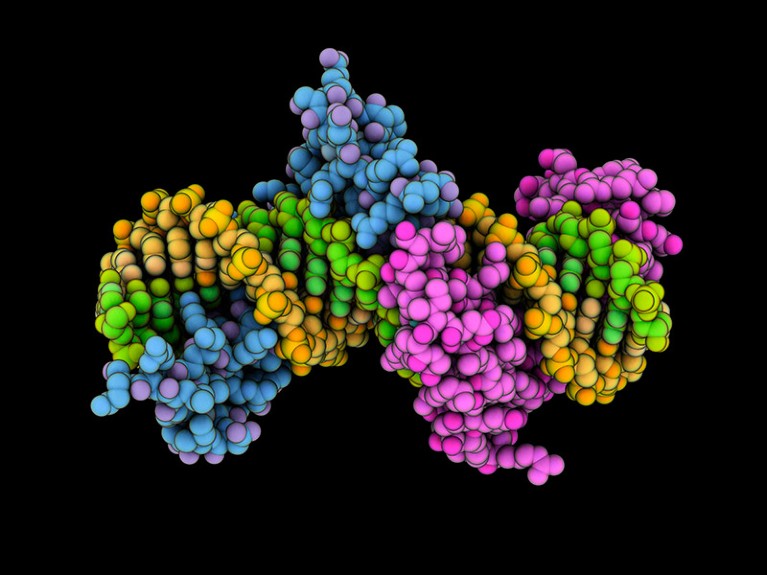
A zinc finger protein (blue and magenta; artificially coloured) in complex with DNA (yellow and green).
Credit: Laguna Design/SPL
An alternative to
genome editing
can reduce the activity of a gene that affects cholesterol levels without changing the DNA sequence — and does so for an extended period, according to a study
1
in mice.
Scientists achieved this effect by changing each animal’s ‘
epigenome
’, one feature of which is a collection of chemical tags that are bound to DNA and affect gene activity. After the treatment, activity of the targeted gene dropped and remained low for the 11 months over which the mice were studied.
The 2023
approvals of the first genome-editing therapy, which relies on
the CRISPR-Cas9 editing system ushered in a new form of medicine that relies on making targeted changes to DNA sequences. But the new findings, published on 28 February in
Nature
,
bolster the case for instead
editing the epigenome
to treat certain diseases, thereby sidestepping some of the
risks that come with breaking and irreversibly altering strands of DNA
.
“This is just the beginning of an era of getting away from cutting DNA,” says Henriette O’Geen, an epigeneticist at the University of California, Davis. “This can alter the expression of genes that are involved in disease — and potentially provide a cure — without changing DNA.”
Mark this gene
Table of Contents
As cells take on new identities during development, the pattern of chemical tags on their DNA often changes. These epigenetic alterations can tell a cell to behave as a liver cell, for example, rather than a brain cell.
Could this one-time ‘epigenetic’ treatment control cholesterol?
After more than a decade of effort, scientists worked out how to modify genome-editing tools to tweak some epigenetic marks. This makes it possible to add a type of chemical tag called a methyl group to DNA at precise locations, for example, to switch a gene off, or to remove methyl groups from a spot in the genome to turn a gene on
2
.
Epigenetic editing’s applications in the clinic were initially unclear, says epigeneticist Marianne Rots at the University Medical Center Groningen in the Netherlands. Researchers were concerned about how specific or effective the approach would be, she says, and how long its effects would last.
A finger on the genome
To address these questions, Angelo Lombardo, a gene-therapy researcher at the San Raffaele Scientific Institute in Milan, Italy, and his colleagues used molecules called zinc finger proteins that, much like the CRISPR–Cas9 system, can be designed to bind to specific sequences in the genome. The team designed a zinc finger protein that could bind to the
PCSK9
gene, which is the target of several existing therapies for high cholesterol. The authors then fused their zinc finger proteins with pieces of three proteins involved in attaching methyl groups to DNA.
UK first to approve CRISPR treatment for diseases: what you need to know
That cocktail of fragments was drawn from a suite of proteins that act during embryonic development, adding methyl groups to ensure that viral sequences lurking in the genome — relics of past infections — are silenced and stay that way for a lifetime. The hope, says Lombardo, was that the long-lasting effects of that natural epigenetic editing would carry over to the gene bound by the zinc finger protein that the authors designed.
Working with mice, the team used this system to edit the
Pcsk9
gene. The animals’ cholesterol levels fell within a month of the treatment. Their levels of the PCSK9 protein also dropped — and stayed low for the 330 days that the researchers tracked them. The effects could last longer than a year, says O’Geen, given that the rodents’ PSCK9 levels showed no signs of rebounding at the end of the experiment.
A rush to epigenetics
The results will add to already burgeoning excitement about epigenetic editing. More than ten companies are focused on developing epigenetic editing therapies, says Rots. A few have reported long-lasting effects in monkeys but have not yet published their findings in peer-reviewed journals.
And Omega Therapeutics, a company in Cambridge, Massachusetts, is conducting a clinical trial of an epigenetic editor that silences
MYC
, a gene that is overactive in many cancers and has been difficult to target using conventional drugs. “It’s exciting to see how things have exploded,” Rots says.
