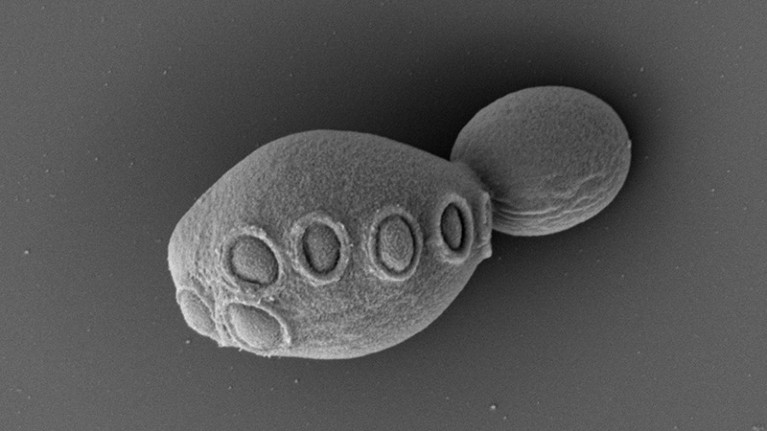
Yeast cells containing 7.5 synthetic chromosomes were able to bud normally, splitting into two cells.Credit: Cell/Zhao et al.
Biologists have produced a strain of yeast whose genome is more than 50% synthetic DNA. Standard brewer’s yeast (Saccharomyces cerevisiae) stores its genetic blueprint across 16 chromosomes. In the new strain, 6.5 of those chromosomes were edited and synthesized in the laboratory — and an extra one was stitched together from edited bits of the yeast’s genetic code.
The feat is the latest milestone for a group of labs, called the Sc2.0 consortium, that has been trying to create a strain of yeast with a fully synthetic genome for 15 years. A package of papers published today in Cell1,2 and Cell Genomics3 describes the team’s accomplishment, how it made the new strain and other tests it performed on the yeast genome.
Scientists downsize bold plan to make human genome from scratch
Some viruses and bacteria have already been engineered to have entirely synthetic genomes, but they have all had simple genetic structures — the bacterium Escherichia coli, for example, has just one chromosome. They have also had a simple inner configuration: the bacteria, for instance, have been prokaryotes, which means they are single cells, without a nucleus to hold their chromosomes. If the Sc2.0 group, which includes researchers from labs in Asia, Europe, North America and Oceania, can achieve its goal, its engineered yeast will be the first eukaryote with a fully synthetic genome. (Eukaryotes are organisms with cells that store their genetic material in a nucleus, and include humans, plants and animals.)
The Sc2.0 team hopes to manipulate its synthetic brewer’s yeast so that it can one day produce drugs and fuels, rather than beer. But the quest has other benefits, says Jef Boeke, a synthetic biologist at New York University in New York City and the leader of the project. By tweaking the organism without interfering with its survival, “we learn a lot about the biology of yeast”, he says.
Nili Ostrov, chief scientific officer at Cultivarium, a non-profit firm in Watertown, Massachusetts, that develops tools for bioengineers, says that Sc2.0 is “pushing the boundaries of what we can engineer in biology”. Historically, genetic engineers have focused on modifying individual genes in organisms. Now, biologists can see what happens when they re-engineer entire chromosomes. “This allows you to ask questions you couldn’t ask before,” Ostrov says. In the process, the project is developing methods that could advance biological engineering, she says.
Rewriting the genome
Table of Contents
One of Sc2.0’s main goals is to eliminate potential sources of instability in the yeast genome. One such source is large stretches of repetitive DNA that don’t code for anything, but that can recombine with each other through natural processes, causing major structural changes in the genome. The synthetic biologists want to have complete control of their engineered yeast, so the team combed through S. cerevisiae’s genome with computer programmes to find highly repetitive regions — and then deleted them. These sequences are effectively “genome parasites”, Boeke says.
Entire yeast genome squeezed into one lone chromosome
Another change the researchers made to reduce instabilities was to remove from the chromosomes all DNA segments that encode transfer RNA, and to relocate them into an entirely synthetic ‘neochromosome’2. Transfer RNAs (tRNAs) are crucial to a cell’s functioning — they ferry amino acids to an apparatus that uses the molecules to make proteins. But the DNA sequences that code for them “are hotspots for instability”, Boeke says. So moving them into their own new chromosome, designed for greater stability, was a way for the team to bring the synthetic yeast under greater control, and probe the limits of biology.
To bring the 7.5 synthetic chromosomes together into a single cell, the team made strains of yeast that each contained one of the edited chromosomes, along with the natural versions of the other 15. Then they bred two of the strains and selected for offspring that contained two different edited chromosomes. Those strains were then bred to add another edited chromosome, and so on.
Even with all the big changes in the chromosomes, the cells that ended up with the 7.5 chromosomes survived and could replicate, Boeke says.
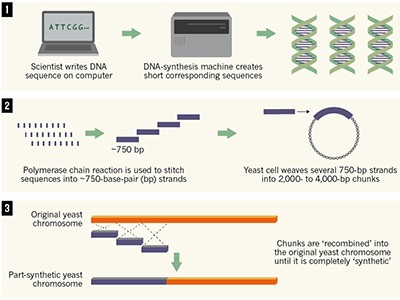
First synthetic yeast chromosome revealed
Although the process of making the cells was time-consuming, what really slowed things down is debugging, Boeke says. Researchers first had to test whether each yeast cell with a new synthetic chromosome in it was viable — meaning it could survive and function normally — then fix any problems by tweaking the genetic code1. When two or more synthetic chromosomes are inside the same cell, this can lead to new bugs that must be fixed, so the debugging problem becomes more complex as the process proceeds.
The Sc2.0 project is allowing scientists to test questions that were previously impossible, Ostrov says — for instance, “What happens when you introduce chromosomes that weren’t there before?”
Boeke says the team is now plugging away at replacing the remaining natural chromosomes with entirely synthetic ones, adding new chromosomes one at a time and then debugging the ever more complex system. “It will take a lot of doing it all over again,” he says.
