
Respiratory syncytial virus in older adults is under-studied owing to a lack of reliable data.Credit: golfcphoto/ Getty Images
When a retired physician in his early 80s checked himself into Rochester General Hospital in New York complaining of severe difficulty breathing, his physicians suspected that his pre-existing heart failure was to blame. They gave him diuretic drugs to try to lower his blood pressure, and started him on antibiotics. The medications, however, made no difference. His physicians were perplexed.
The patient’s daughter happened to work at the hospital herself as a paediatrician who specializes in respiratory syncytial virus (RSV), a virus that infects 33 million children under the age of five globally every year1. She knew Edward Walsh, head of infectious diseases at the hospital, and she asked him to drop by her father’s room to see if he could work out what was wrong. After a brief examination, Walsh made a surprise announcement: RSV could be the culprit.
Part of Nature Outlook: Respiratory syncytial virus
Tests revealed that Walsh was correct. This was both good and bad news — bad because treatments do not exist for adult RSV infections, but good because the patient’s physicians could now stop the other unnecessary medications they had put him on.
Fortunately, the patient recovered on his own, but his story is “a great example” of a broader, problematic trend in medicine, says Ann Falsey, a physician who specializes in infectious diseases at the University of Rochester Medical Center in New York and who works closely with Walsh. “People just don’t even consider an RSV diagnosis in adults.”
RSV is one of the viruses that cause common colds, as well as bronchitis and pneumonia, in older people and health-compromised adults. Yet, even among physicians, RSV remains stubbornly known as a mainly paediatric pathogen, says Falsey. This is partly because as people get older their immunity develops and their airways get larger, so RSV usually becomes a non-issue, says Harish Nair, an epidemiologist at the University of Edinburgh, UK, who specializes in the virus. “We get a runny nose and some sniffles and that’s pretty much it.”
However, in adults with underlying conditions, such as diabetes or heart or lung disease, RSV can pose a significant risk (see ‘Rising risks’). In a 2022 meta-analysis2 of 14 studies of RSV occurrence in US adults over the age of 65, for example, researchers found that people with chronic medical conditions were 1.2 to 28 times more likely to be hospitalized than were adults without an underlying condition.
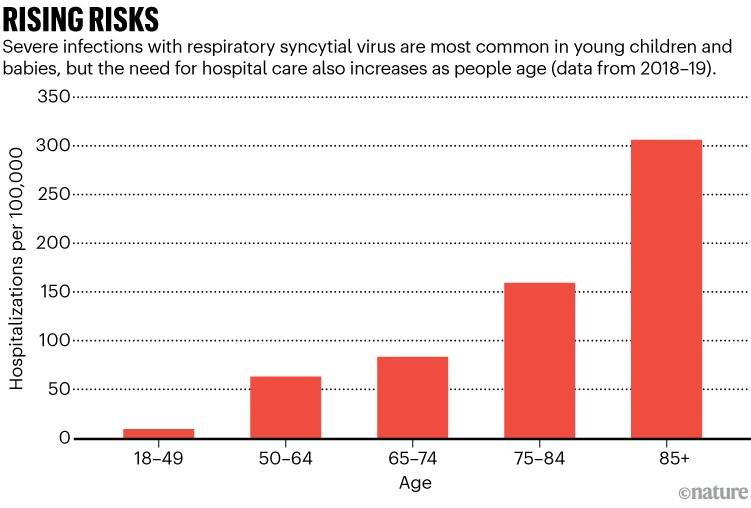
Source: A. R. Branche et al. Clin. Infect. Dis. 74, 1004–1011 (2022)
The risk of serious illness from RSV also begins to climb as people age. Each year, RSV causes about 159,000 hospitalizations, 119,000 emergency-department visits and 1.4 million outpatient appointments in older adults in the United States2. Fatality rates hover at around 6–8% in those who are hospitalized, meaning that somewhere between 9,500 and 12,700 older adults in the United States die from RSV every year. A 2005 study3 of both older and high-risk adult patients in the United States — the largest and most comprehensive study of its kind, according to Falsey — evaluated a total of 2,514 illnesses and put the RSV mortality estimate higher, at around 14,000 annual deaths.
Even fewer studies have been conducted outside the United States. But in a paper released this year4, Nair and his colleagues counted about 787,000 RSV hospitalizations and 22,000–47,000 deaths in adults over 65 years old in high-income countries around the world. These figures turned out to be about 2.2 times higher than previous estimates.
Despite all these data, RSV still “hasn’t been widely perceived or recognized as a cause of serious respiratory illness in adults”, emphasizes Edward Belongia, an infectious-disease epidemiologist at the Marshfield Clinic Research Institute in Wisconsin.
However, the impacts of RSV on adults could soon be significantly lessened. Two vaccines for adults gained US Food and Drug Administration (FDA) approval in May — both show high degrees of efficacy. Two more promising vaccines are in the pipeline. “This is really revolutionary,” Belongia says.
Overlooked burden
Table of Contents
Protecting adults from RSV will require raising awareness, especially among physicians, because vaccinations in general are strongly driven by recommendations from health-care practitioners, says Helen Chu, a medical doctor who specializes in viral respiratory infections at the University of Washington in Seattle. “If health-care practitioners aren’t aware of RSV as an adult pathogen, they will not recommend the RSV vaccine.”
RSV in adults is under-recognized and understudied, in part because of a difficulty in sourcing reliable data for this group. Some of this comes down to biology. For one, RSV infections in adults are re-infections. And “younger kids have a lot of snot, full of virus”, Nair says. Adults already have partial immunity, so the number of viral particles that turn up in their noses are not nearly as high as that in children. Adults also shed viral particles for a shorter period of time.
As a result, nasal-swab-based rapid antigen tests for identifying RSV in adults “are nearly useless”, Falsey says. Around 1,000 viral particles per millilitre are needed to generate a positive RSV antigen test result, but “most adults don’t have that in their nose”, she says.
Polymerase chain reaction (PCR) tests are much more reliable for detecting RSV in adults, correctly identifying infections as much as 93% of the time5.
PCR testing — or any sort of testing at all — is still under-used. A study released this year6 revealed that just 4.3% of 937 US hospitals tested people aged 65 or older who were hospitalized with lower respiratory tract infections for RSV. The testing oversight largely boils down to a lack of awareness, Falsey says, mainly because the last time most non-paediatric physicians thought about RSV “was in medical school”.
There are further reasons for the data deficiency, too. In some cases, even if a test does come back positive for RSV in a person who has been hospitalized for a respiratory illness, hospitals might not list RSV as the cause, Chu says. In a 2017 paper7, for example, Falsey and her colleagues reviewed 110 RSV-related adult hospital admissions, but found that just 6% of the medical records listed RSV as the primary diagnosis, and only 51% mentioned RSV at all. By contrast, data that the team examined from 188 people admitted with influenza showed that influenza was listed as the primary diagnosis for 30% of these patients, and was noted in 79% of the total medical records.
It doesn’t help, either, that there’s no effective antiviral treatment for RSV for adults, Falsey adds. “Hospitals and doctors don’t want to spend $50 on RSV testing if there’s nothing they can do.”
But thorough testing and record keeping are important for understanding true rates of RSV infection in adults and for raising awareness. Such data can potentially help physicians to cut down on prescribing unnecessary antibiotics, but more than that, Falsey says, testing can hammer home the significance of RSV to physicians in a way that research papers cannot. “If they haven’t seen one or two of their patients get seriously ill, it doesn’t feel personal,” Falsey says. “I want people to do a fair amount of testing so they understand the true burden.”
The good news on the awareness front is that some multiplex PCR tests now automatically include flu, RSV and SARS-CoV-2. Physicians and hospitals have also stepped up their viral testing in general since the pandemic. “It’s funny, people will come to me now like, ‘My goodness! What is going on with RSV? It’s in all my old people!’” Falsey says. “I’m like, ‘Nothing is going on — it’s just that you’re testing.’”
A vaccine’s journey
Two RSV vaccines have now completed phase III trials and could be available by the end of 2024. The first of these vaccines is manufactured by the UK pharmaceutical company GSK. The vaccine showed efficacy of nearly 83% in preventing RSV infection in all adults over 60 years old, and of nearly 95% in those with at least one underlying medical condition. The second vaccine, developed by the pharmaceutical firm Pfizer, based in New York City, demonstrated similar levels of efficacy, at nearly 86% for adults over 60.
Furthermore, biotechnology company Moderna in Massachusetts is developing an mRNA-based RSV vaccine, and Bavarian Nordic, a biotechnology company based in Hellerup, Denmark, is creating an adenovirus vector RNA vaccine. “We’re probably going to be spoiled for choice,” Nair says.
A continued effort
Even after these vaccines are available, some unknowns will remain about RSV infection in older adults, Belongia says. This includes questions about rates of RSV infection, the risk of an older adult developing severe illness and the potential benefit of vaccination for people in this group. No vaccine is 100% effective — and not every person who is eligible will get vaccinated — so efforts are still needed to develop targeted treatments. In low- and middle-income countries — where most of the world’s population lives — huge data gaps also need to be filled. Older people in these countries tend to receive less medical care and attention in general, Nair says, because “funders see the paediatric age group as a priority”.

More from Nature Outlooks
Regardless of the country, what’s ultimately needed across the board for adult RSV is more awareness, says Nair, who is leading a European consortium called Promise. Promise is a public–private partnership with 22 members, including universities and pharmaceutical companies. The group is working to shed light on the RSV disease burden in older adults in Europe and at the global level, with the aim of providing data that can guide decision makers to the most effective path for vaccine introduction. The Promise team is also now working on a cost-effectiveness analysis for RSV vaccines in older adults and is trying to gain a better understanding of the risk factors that lead to poor outcomes in those with RSV in this age group. Funding is one of the main barriers to answering these questions, Nair says, as is “poor awareness” of RSV’s prevalence and impact on older people.
“RSV is not a known name,” Nair reiterates. “The important thing is that there are going to be vaccines that will be available, but whether or not these vaccines get recommended as part of immunization in this age group — that’s going to be really key.”
