The standard model of particle physics has the curious underlying feature that no microscopic phenomena are predicted to change when they are hypothetically transformed in certain ways. One of these fundamental constraints is called CPT symmetry. It implies that, if all of the matter in the Universe were simultaneously replaced with antimatter and transformed into its mirror image, and the flow of time reversed, then the resulting hypothetical universe would be indistinguishable from our own at the microscopic level. The equivalence principle underlying Albert Einstein’s general theory of relativity further predicts that matter and antimatter fall to earth with the same acceleration.
These two constraints are so fundamental that it would be difficult to formulate a consistent understanding of nature without them. Nevertheless, it is worth testing whether they really hold up in ultra-precise measurements carried out using the most modern technologies, because any deviation, however small, would force scientists to radically rethink the basis of our theories of physics. Writing in Nature, Baker et al.1 (members of the ALPHA collaboration) report a major step towards this goal. They have slowed down atoms of antihydrogen — the antimatter counterpart of hydrogen — to unprecedentedly low velocities by bathing them in a beam of ultraviolet laser light. This could allow measurements of the atoms to be made with exceptionally high precision.
Antihydrogen is the simplest stable atom that consists only of antimatter particles, namely an antiproton and an antielectron (a positron). Measurements of antihydrogen therefore provide an ideal way to test the symmetry between matter and antimatter, but such experiments present formidable obstacles. In 1995, 11 antihydrogen atoms were produced from reactions in a particle accelerator at CERN, Europe’s particle-physics laboratory near Geneva, Switzerland, and hurtled down a 10-metre-long vacuum tube at nine-tenths of the speed of light2. Each atom existed for barely a few tens of nanoseconds before being destroyed by striking a particle detector.
Much of the ensuing research into antihydrogen has involved inventing new ways of producing samples of increasingly slower-moving atoms. This was eventually achieved by confining and mixing clouds of antiprotons and positrons in magnetic fields that acted as ion traps to produce antihydrogen atoms. The atoms were then confined by another complex configuration of magnetic fields that acted as a neutral-atom trap3,4. The ALPHA collaboration at CERN’s Antiproton Decelerator facility can now routinely trap 1,000 antihydrogen atoms for many hours in this way. This has allowed an atomic frequency of antihydrogen, which corresponds to the energy of a characteristic atomic transition, to be measured5 with a fractional precision of 2 parts in 1012. No deviation from the corresponding frequency of hydrogen was observed, which is exactly the outcome expected from CPT symmetry.
A major limitation in these experiments arises from the fact that, although the studied antihydrogen atoms are vastly slower than the first such atoms produced 25 years ago, they still move randomly within the magnetic trap, at velocities of up to 300 kilometres per hour. Samples of slower atoms are needed to achieve even higher precision, and to facilitate future experiments probing the gravitational free-fall of antihydrogen. Similar problems affect some proposed designs for quantum computers6, in which trapped ions must be held nearly stationary before they can be manipulated with lasers to store quantum bits of information.
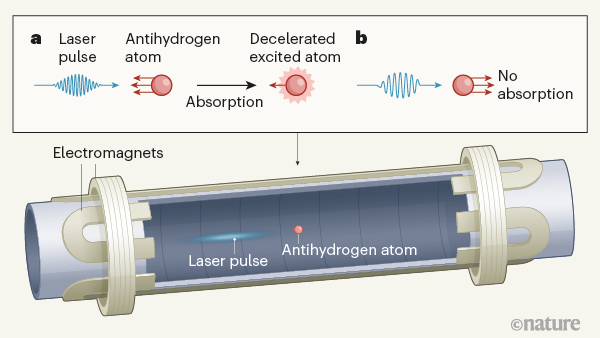
Metastable antiprotonic helium atoms, in which one of the two electrons of a helium atom has been replaced by an antiproton, have been slowed down simply by immersing them in a gas of colder matter7. But this approach would not be practical for antihydrogen atoms because they would immediately annihilate in a matter–antimatter reaction. By contrast, a beam of light can exert a mechanical force on both matter8–13 and antimatter, without causing the latter to annihilate. A handheld red or green laser pointer typically emits between 1015 and 1016 laser photons per second, but the momentum that each photon can impart is so minuscule that it is hard to detect the pressure exerted by this light on everyday objects. The mass of a single antihydrogen atom, however, is so small (1.7 × 10–24 grams) that its velocity can be changed by about 12 kilometres per hour each time it absorbs a laser photon that has an ultraviolet wavelength of 121.6 nanometres.
In their current study, Baker et al. used a laser carefully tuned to a wavelength that causes only trapped antihydrogen atoms that move towards the laser to absorb photons and slow down (Fig. 1). The absorption selectivity arose from a type of Doppler effect8,9 experienced by the atoms — an effect that caused the light of the laser beam to seem to shift towards shorter wavelengths. The shifted wavelength exactly matched the photon energy needed to be absorbed by the atoms, and this absorption promoted the atoms from their ground state to an excited state. The atoms then spontaneously returned to the ground state by emitting another photon in a random direction. The authors observed that a few dozen such absorptions slowed down a fraction of the atoms in the sample to below 50 kilometres per hour. This reduction in speed corresponds to a cooling of the atoms.
Conversely, the atoms that moved away from the laser experienced the opposite Doppler effect: the wavelength of the light apparently shifted further away from that needed for photon absorption. The light therefore passed right through the receding atoms, thus avoiding their undesirable acceleration. Once the antihydrogen atoms had been properly cooled, the authors irradiated them with a pair of counter-propagating laser beams to excite a characteristic atomic transition. Because of the low velocity of the atoms, the line that corresponds to this transition in the atomic spectrum was four times sharper than had been observed for atoms without laser cooling. This will allow researchers to carry out future comparisons of the characteristic atomic transitions of hydrogen and antihydrogen at a higher precision than was previously possible.
One limitation of the reported method is that it is difficult to generate 121.6-nm laser light of sufficient intensity to cool the antihydrogen atoms efficiently. Baker and colleagues used a train of laser pulses of nanowatt-scale average power, which meant that many hours were required for each atom to absorb the dozens of photons needed for substantial cooling. The authors plan to increase the laser power in future experiments to speed up the process. Another approach might be to use continuous, rather than pulsed, laser beams14.
Finally, because laser cooling leads to a greater concentration of slower atoms at the magnetic-field minimum of the neutral atom trap, it might allow denser clouds of antihydrogen to be produced than is currently possible. This would further improve the precision of measurements in future experiments.