It is almost a century since insulin was first used to treat diabetes1. Since then, a great deal has been learnt about the complex metabolic pathways that are regulated by insulin and the related molecule insulin-like growth factor 1 (IGF1), acting through receptor proteins (reviewed in ref. 2)2. But it is less clear how the activity of these receptors is regulated in the cells that actually produce insulin, the pancreatic β-cells. Such knowledge is urgently needed because reduced β-cell function is a key contributor to diabetes. Deciphering the molecular pathways that regulate β-cells might therefore help to better manage, or even prevent, this disease. Writing in Nature, Ansarullah et al.3 identify a previously unknown regulator of β-cells, and outline the mechanism by which this protein can ‘tailor’ expression of the insulin receptor.
First, the authors analysed levels of messenger RNA in mouse cells, to identify genes that were highly expressed specifically in the embryonic pancreas. This revealed an abundantly expressed mRNA encoded by a gene on chromosome 3. The corresponding human gene is named oestrogen-induced gene (EIG121), and the mouse and human proteins are highly evolutionarily conserved.
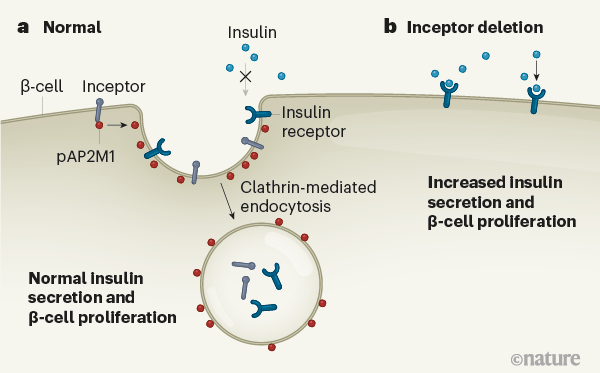
Ansarullah et al. renamed the protein insulin inhibitory receptor (inceptor), because of its similarities to the insulin and IGF1 receptors. All three receptors span the cell membrane and have similar extracellular domains. But, unlike the insulin and IGF1 receptors, the short cytoplasmic tail of inceptor carries an amino acid sequence known to bind to the assembly polypeptide 2 (AP2) protein complex. AP2 is involved in a process called clathrin-mediated endocytosis, through which molecules and receptors at the cell surface are transported into the cell.
Then, the authors examined the function of inceptor by generating mice completely lacking the inceptor gene, and mice in which the gene could be deleted specifically in β-cells. The two models show generally similar traits. These include low glucose levels in the blood, along with enhanced insulin secretion compared with controls in the period immediately after an increase in blood glucose levels (after a meal, for instance, known as first-phase secretion), and a higher capacity to respond to increased blood glucose levels (improved glucose tolerance). The idea that inceptor acts mainly in β-cells is supported by the observation of greater β-cell proliferation and mass in both strains of mutant animal compared with controls, indicating that the mutant β-cells contain more insulin. And when the pancreatic tissues rich in β-cells were grown in culture, those of the mutant animals showed higher activity of the protein p-Akt, which is activated by insulin/IGF1 receptor pathways, than did the controls. Together, these data indicate that inceptor acts to directly calibrate the expression of the insulin receptor and thereby contributes to maintaining healthy glucose levels.
These findings are complementary to scientific reports from several decades ago, which argued that insulin has a physiological role in β-cells4,5. Indeed, deletion of the insulin receptor in β-cells (a modification dubbed βIRKO) in mice leads to traits5,6 that are the opposite of those seen in Ansarullah and colleagues’ β-cell-specific mutant mice. For example, βIRKO mice show a blunted first-phase secretion leading to glucose intolerance5, which arises owing to effects specifically in the mutant β-cells6. The data also align with several studies using ex vivo and in vitro models that demonstrate a direct role for insulin signalling in β-cell biology7–11. β-cells lacking functional insulin or IGF1 receptors, or lacking other proteins regulated by these receptors, manifest diverse defects, including reduced expression of the protein PDX1, which controls β-cell maturation and secretory function. Lowered PDX1 expression leads to altered growth of β-cells and blunted glucose-stimulated insulin secretion2,6,12–15.
Ansarullah et al. went on to further investigate whether inceptor might densensitize β-cells to insulin through its interactions with AP2. They found that inceptor interacts with pAP2M1, the active form of the AP2M1 subunit, to aid clathrin-mediated endocytosis of the insulin and IGF1 receptors. Once in the cell, they cannot be activated by insulin (Fig. 1).
The fact that inceptor can desensitize β-cells to insulin in this way has implications for questions that have dogged the field for decades: what are the levels of insulin that surround human β-cells in the fasting and post-feeding states, and how is insulin-receptor signalling in β-cells affected in diabetes, when cells across the body fail to respond to insulin? It is sometimes assumed that local insulin levels around β-cells must be high, but there are virtually no experimental data that confidently provide the precise dynamic concentrations of insulin at the surface of the β-cells in vivo in mammals.
A related mystery is the location of the insulin receptor on β-cells. If it is located at the cell’s basolateral surface, it would be close to systemic vessels, enabling it to be activated by insulin circulating in the blood. By contrast, its presence at the opposite, apical side might mean it was modulated by insulin secreted from surrounding β-cells (known as paracrine regulation). A basolateral position is supported by the observation that injected insulin has direct beneficial effects on human β-cells16, and that these benefits are lost in people who have type 2 diabetes16,17. The identification of inceptor should now prompt fresh studies to re-examine the potential role of insulin in paracrine regulation.
Although the identification of inceptor undoubtedly advances our understanding of insulin signalling, several questions remain. For example, how do metabolic regulator molecules such as hormones, metabolites and stressors affect the expression and function of inceptor in β-cells, as well as the hypothalamic–pituitary–gonadal axis that controls reproduction and immunity, tissues of which also express inceptor? Are there genetic variants in or near the inceptor gene that are associated with diabetes or metabolic diseases? Insulin signalling has been shown to directly18 and indirectly19 regulate gene expression — it would be worth contemplating how interactions between inceptor and insulin receptors might alter these nuclear effects of insulin signalling. Finally, changes in the patterns in which methyl groups are added to mRNA have been found in β-cells in people who have type 2 diabetes20. What part might modifications of inceptor mRNA play in altering β-cell biology?
The identification of inceptor cements the legacy of insulin action in β-cells. The protein’s discovery warrants a renewed focus on finding ways to harness proteins in the insulin signalling pathway in β-cells, with the long-term goal of more effective management of — or even a cure for — diabetes.