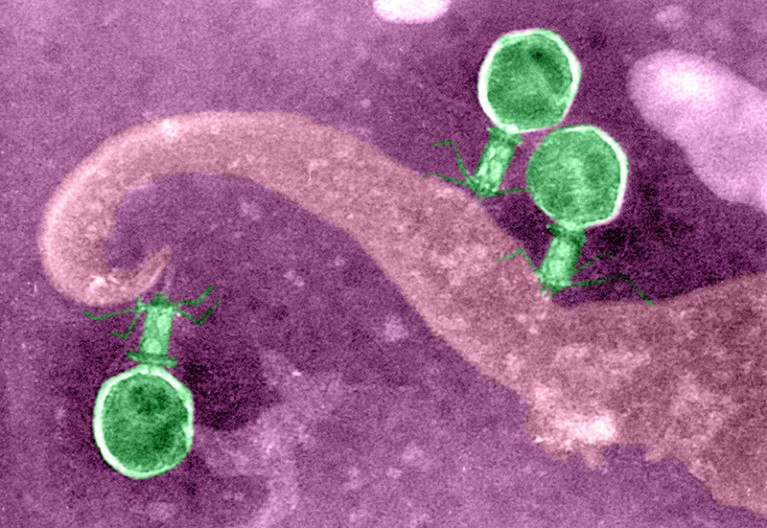
Phage viruses (green) inject their genes into bacteria to replicate themselves.Credit: Biophoto Associates/SPL
The Good Virus: The Untold Story of Phages: The Most Abundant Life Forms on Earth and What They Can Do For Us Tom Ireland Hodder Press (2023)
The world is teeming with viruses: they flourish in water, soil and our guts. In the wake of the COVID-19 pandemic, it is safe to say that viruses are unlikely to win any popularity contests. Yet, in The Good Virus, science writer Tom Ireland attempts to improve their reputation.
This engaging book highlights the brighter side of the viral world by focusing on one group of viruses, called bacteriophages (phages), that infect microorganisms such as bacteria and archaea. Society tends to overlook phages; the viruses that grab our attention typically belong to a cohort of disease-causing terrors, such as SARS-CoV-2, HIV and Ebola. But the stigma can be lifted, argues Ireland, if researchers succeed in developing phages that can treat infections by deadly, drug-resistant bacteria.
Researchers — aided by citizen scientists — are already combing through phages collected around the world for the best medical candidates. There is no shortage of material: one litre of water can contain billions of the viruses, and they promote the release of an estimated three gigatonnes of carbon from infected bacteria into the seas each year.

Why did the FBI track Nobel-winning microbiologist Salvador Luria?
Phages are also laboratory darlings that have had an outsize impact on our understanding of genetics and molecular biology. The legendary and elegant 1950s experiments by Martha Chase and Alfred Hershey that established DNA — not protein — as hereditary material were performed using phages (A. D. Hershey & M. Chase J. Gen. Physiol. 36, 39–56; 1952). The first full genome to be sequenced was from a phage.
But even those who study phages tend to focus on only a handful of viruses. The mystery surrounding the overlooked majority makes some sections of the book a delight. To learn more about phages is to discover fascinating details about a hidden world in which they mould microbial ecosystems and accelerate evolution by swapping genes with their hosts. Engineered phages could one day deliver drugs to the brain and might even be incorporated into antibacterial building materials used in hospitals.
A historical, yet futuristic, therapy
Table of Contents
Ireland devotes much of the book to discussing the potential merits of phages as medicines for infecting and killing drug-resistant strains of bacteria. The idea has become more appealing as the problem of drug resistance has grown. By some estimates, 10 million people will die each year from antibiotic-resistant infections by 2050. It is a future as unjust as it is grim: as Ireland notes, “up to 90% of those deaths are predicted to occur in Africa and Asia”.
Phage therapy, some say, could allow us to avoid that outcome. If these viruses can be harnessed as a treatment for infection, administering a mixture of phages — each with its own way of infecting bacterial cells — could slow the emergence of resistance. The viruses themselves are capable of change and could evolve ways to fend off a bacterium’s resistance mechanisms as they develop.
CRISPR tools found in thousands of viruses could boost gene editing
It is an idea both futuristic and historical: some countries — particularly former members of the Soviet Union — have used phage therapy to treat infections for nearly a century. Some of these labs foundered in the immediate aftermath of the fall of the Soviet Union, when countries such as Georgia faced a period of economic instability. Ireland offers riveting accounts of a phage lab in Tbilisi that had a staff of hundreds and supplied tonnes of phage medicines each year at its peak. The researchers who remained after the Soviet Union’s collapse struggled, in the 1990s, to provide some phage therapies, amid electricity outages, rusting equipment and a shortage of basic supplies. Since then, a few of these labs have bounced back.
A risky endeavour?
Yet most Western doctors and scientists have rejected the approach. Prejudice has no doubt contributed to that scepticism, but there are real logistical hurdles to deploying phage therapy. Despite dramatic anecdotes of lives saved by last-minute interventions, the data simply do not yet exist to support the widespread use of such treatments.
Beyond coronavirus: the virus discoveries transforming biology
Here, Ireland walks a knife edge: although positive one-off stories hint at a possible way to save more lives, they also run the risk of giving false hope to people in desperate circumstances. Yes, prejudice has held the field back and yes, drug regulation can slow the development of therapies in ways that can be galling when someone’s life is on the line. But it is also true that regulators have reasons to be concerned.
Phage therapy carries risks. If a preparation contains too much material from the original bacterial host, it can trigger a deadly immune response. And little is known about how phages could affect the microbial ecosystems in our bodies as they take up residence and swap genes with their neighbours. There are, for example, known cases of bacteria that become human pathogens only after they are infected with specific phages (P. L. Wagner & M. K. Waldor Infect. Immun. 70, 3985–3993; 2002).
The Good Virus is timely. Excitement about phage therapy is growing: biotechnology firms are jockeying for position; key clinical trials are finally getting under way; and phage production in Tbilisi and elsewhere is flourishing once again. It’s an exciting time for a field that has, for too long, been unfairly overlooked. But there is still a long way to go before we know whether phages will spare us from the drug-resistant nightmare on the horizon and cast the viral world in a better light.
