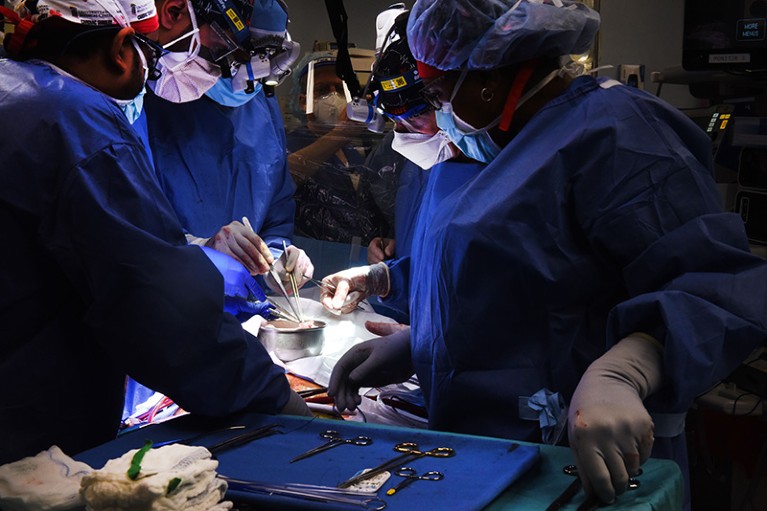
A team at the University of Maryland Medical Center transplanted a pig heart into a living person for the first time in January 2022.Credit: University Of Maryland School Of Medicine/ZUMA
David Bennett’s heart was failing. The 57-year-old handyman from Maryland had weeks to live and, because he had a history of failing to follow treatment instructions, he wasn’t eligible for a transplant.
Not one from a human, anyway.
In January, Bennett’s doctors offered him the chance to receive a heart from a pig. He took it. “I know it’s a shot in the dark, but it’s my last choice,” he said in a press release from the University of Maryland Medical Center in Baltimore, where he was being treated. On 7 January, doctors transplanted the heart, which had been genetically modified so that the human body would tolerate it.
Bennett survived for eight weeks with his new heart before his body shut down. After his death, the research team learnt that the transplanted organ was infected with a pig herpesvirus that had not been detected by tests1.
But even a few weeks is a long time for an animal organ placed in a human, known as a xenotransplant. Given that the human immune system begins attacking non-genetically modified pig organs in minutes, other xenotransplantation researchers are impressed with the experiment. “It’s actually beyond my expectation that the patient lived up to two months,” says Luhan Yang, a bioengineer and chief executive of Qihan Biotech in Hangzhou, China. “I think it’s a victory for the field.”
It was just one of several cases of xenotransplantation that made the news this year. A few months after Bennett’s procedure, two research groups2,3 independently reported transplanting the first pig kidneys into three people who had been declared legally dead because they lacked brain function. The trials found that the organs produced urine and were not rejected by the human immune system, even two to three days after the procedure. Surgeons performed two more pig-heart transplants in brain-dead people in June and July.
Clinical trials for pig-to-human organ transplants inch closer
Many researchers expect that these early efforts will soon lead to a surge in small clinical trials of xenotransplantation in extremely ill people. Proponents say that such efforts, if successful, could help to make a large dent in the list of the thousands of people in need of organ transplants (there are more than 100,000 in the United States alone), many of whom will die while waiting. For now, researchers say they are waiting for regulators such as the US Food and Drug Administration (FDA) to evaluate several applications that have been submitted. An FDA spokesperson says that the agency does not comment on the status or existence of applications.
In June, the agency held a meeting to address the growing number of US research teams that want to start formal clinical trials involving xenotransplants. Armed with data from hundreds of baboons that have survived for up to three years after receiving pig organs or cells — mostly hearts, kidneys and insulin-producing islet cells — scientists tried to convince agency officials that xenotransplantation is ready for human use.
Clinical trials, researchers argue, are needed to answer questions such as the best type of pig to use and how to ensure the animals are not carrying infections. “I think we need to take that step forward and go to the clinic,” says Wayne Hawthorne, a transplant surgeon at the University of Sydney in Australia.
Bennett’s transplant and subsequent death brought unprecedented public attention to the topic — but it also exposed the risks. Researchers see the need to move cautiously. “If there’s a problem, you could set the whole field back,” Hawthorne says.
Animal sources
Table of Contents
Xenotransplantation has long been a dream of transplant surgeons, who are faced with a critical shortage of suitable organs. In the 1960s, researchers began testing chimpanzee and baboon organs in humans with limited success, but raising enough animals for transplants would be impractical.
Pigs could provide a more reasonable source of organs, because they are closer to humans in size and anatomy, and are already produced in mass quantities for agriculture. Pig organs might even have some advantages over human equivalents. Surgeries could be scheduled in advance and organs used fresh, rather than requiring that a patient and a surgical team be available at a moment’s notice when a genetically compatible donor dies.
Furthermore, surgeons might not know a human donor’s history of disease or genetic predispositions. “When we screen donors, we do it for an hour because we don’t have longer,” says Jay Fishman, an infectious-disease specialist at Massachusetts General Hospital in Boston. With pigs, he says, “we have the opportunity to do screening we don’t do in humans”.
Next steps for the xenotransplantation of pig organs into humans
Until the early 1990s, pig organs came with a big problem: the human immune system rejected them. Transplant surgeon David Cooper at Massachusetts General Hospital found a solution when he discovered4 that the immune systems of humans and other primates were reacting mainly to one sugar molecule on the surfaces of pig cells, called α-Gal. Mutating a pig gene encoding a protein that helps to make the sugar prevents cells from producing α-Gal, allowing organs transplanted from these modified animals to survive for much longer in non-human primates.
The advent of the gene-editing technology CRISPR–Cas9 in the 2010s provided a catalyst for the field, making it easier to modify not only the gene involved in α-Gal production, but a host of others that might help human bodies to tolerate pig organs. Several companies are developing pig organs with different modifications; none has yet been approved for xenotransplants beyond the limited trials done so far.
The pigs used in Bennett’s transplant, which were made by the company Revivicor in Blacksburg, Virginia, have ten genetic modifications. The company altered four pig genes, including one that helps to grow pig organs to an appropriate size for the human body, and added six human genes: four that suppress the immune response and two that prevent blood from coagulating owing to inflammation.
Other teams have used slightly different approaches. For instance, Makana Therapeutics, headquartered in Miami, Florida, has modified only three genes in its pigs. The changes in these genes all prevent human antibodies from attacking an organ, and they show the most evidence of improving organ survival in non-human primates, says company founder Joe Tector. “As genetic engineering gets better, it will be more straightforward and feasible to talk about adding DNA or swapping DNA,” he says.
Eckhard Wolf, a molecular biologist at Ludwig Maximilian University of Munich in Germany, agrees. “Our general strategy is to keep it as simple as possible,” he says. His team has engineered five genetic modifications into a wild breed of mini pig from New Zealand, and the first litter was born in September. These mini pigs don’t need a modification to limit their growth because their organs are naturally similar in size to those of humans. If all goes well when his team transplants the mini-pig hearts into baboons, Wolf says, the European Medicines Agency, which regulates drugs and medical products, could approve small human trials of hearts within three years.
Trial planning
With human studies only just beginning, each genetic modification has been tested and optimized exclusively in non-human primates — and some species, for unknown reasons, tend to create more antibodies against pig organs than humans do. It’s unclear which, if any, of the modifications will prove crucial for transplants into humans.
Despite the differences, some research teams say they’ve been asked by the FDA to provide more data on how pig organs fare in non-human primates. “It’s like saying a drug suggests it will do what we want in humans but won’t in monkeys — but let’s still test it in monkeys,” says Cooper. “It’s completely illogical to be doing that.” The FDA did not comment on how much primate data would be needed for specific cases, but has issued guidance saying that primate models would not be sufficient to establish that pig organs are safe for humans.
Transplants into brain-dead people could provide an intermediate step. Surgeon Robert Montgomery at New York University, who led one of the teams that implanted a kidney this year3, is planning to do more transplants in brain-dead people before applying for clinical trials involving living individuals. The Revivicor pigs used in his experiment had only one genetic modification, involving α-Gal; the FDA approved pigs with this alteration in December 2020 for human consumption and for some medical uses other than transplants. Montgomery worries that a complex combination of modifications could have unpredictable interactions.
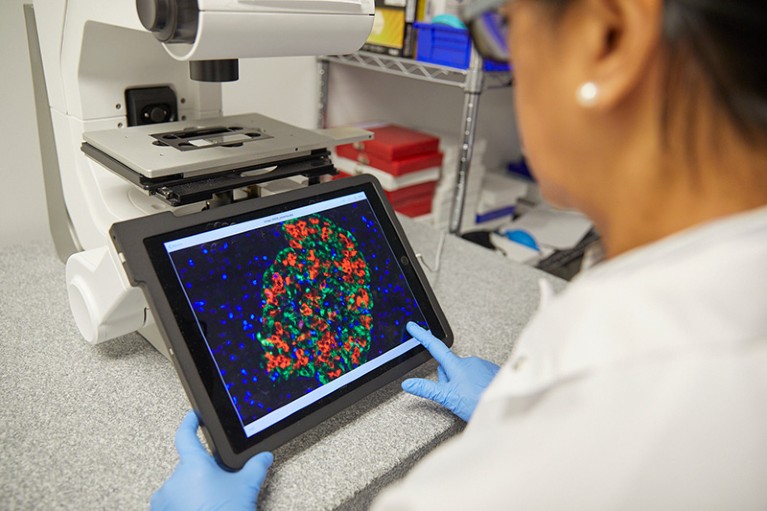
Biotech firm eGenesis has engineered animal islet cells that can be transplanted into humans.Credit: Ken Richardson for eGenesis
Other researchers think that trials in living people will be the best way to determine whether the body rejects the organ months after the transplant. Keeping brain-dead individuals on life support for this long could be considered unethical, says Jayme Locke, a transplant surgeon at the University of Alabama at Birmingham who led the other team that transplanted a kidney this year into a brain-dead person2. Locke is working towards applying to the FDA for permission to start clinical trials using kidneys from the same Revivicor pigs as used for Bennett’s heart. Her team has already listed one such trial in a federal registry, although it is not yet approved and has yet to begin recruiting the 20 patients who would receive the kidneys.
Other tissues, such as insulin-producing islet cells, might not trigger much of an immune reaction at all, and a simpler set of genetic modifications might be sufficient, Hawthorne says. His team has engineered a line of pigs for islet-cell production that lack α-Gal and contain two extra genes that dampen the human immune response. In June, his team reported5 that the transplanted islets had cured diabetes in five baboons, which lived for nearly two years without needing insulin or immunosuppressive drugs.
Hawthorne is now planning a clinical trial of the pig islet cells in people who have a severe form of type 1 diabetes that causes blood sugar to drop extremely suddenly. If his team can find the funding, he says, trials could begin within a year if they are approved. Other groups have previously found that pig islet cells seem to be safe in humans6.
Disease-proof pigs
One of regulators’ greatest concerns about the technology is the presence of transmissible diseases in pigs. It’s not clear how much of a problem this poses, but there are several ways infections could cause issues.
Diseases that affect pigs could jump from transplanted organs to humans. Such concerns caused Qihan Biotech to halt xenotransplantation efforts earlier this year after an outbreak of African swine fever in China. That prompted Yang and her colleagues to genetically engineer a pig that is resistant to the disease. The manuscript describing their work is currently under review.

Next steps for the xenotransplantation of pig organs into humans
Then there are porcine endogenous retroviruses (PERVs), viral elements embedded in the pig genome. These are not picked up from the environment, but are pieces of inherited viral DNA. They are harmless to pigs, but studies disagree on whether they could jump from an organ into human cells, and if they might be harmful to people or to the pig organ. “It’s too early to understand whether it’s a real concern or a hypothetical,” Yang says.
Nonetheless, to see whether it was possible to inactivate these viral elements, Yang and George Church at Harvard Medical School in Boston, Massachusetts, used CRISPR to scramble all known PERVs in the pig genome: a total of 62 modifications in a pig kidney cell line, a record for gene editing7.
“There was disbelief that you could make that many edits and produce viable donors,” says Michael Curtis, chief executive of the biotech firm eGenesis in Cambridge, Massachusetts. Church and Yang founded eGenesis to commercialize CRISPR technology for xenotransplantation, and one of its macaques with a transplanted kidney from a pig with dozens of PERV knockouts has survived for nearly a year. The company is now using this technology to create pig hearts, kidneys and livers with up to 80 edits in all, depending on the organ’s intended purpose.
Even getting rid of PERVs and protecting against known pathogens wouldn’t prevent an unfamiliar virus from causing havoc, says Fishman. “There are still unknown pathogens that might come from pigs,” he says. A person with a xenotransplanted lung, for instance, might be able to get a respiratory virus that infects only pigs.
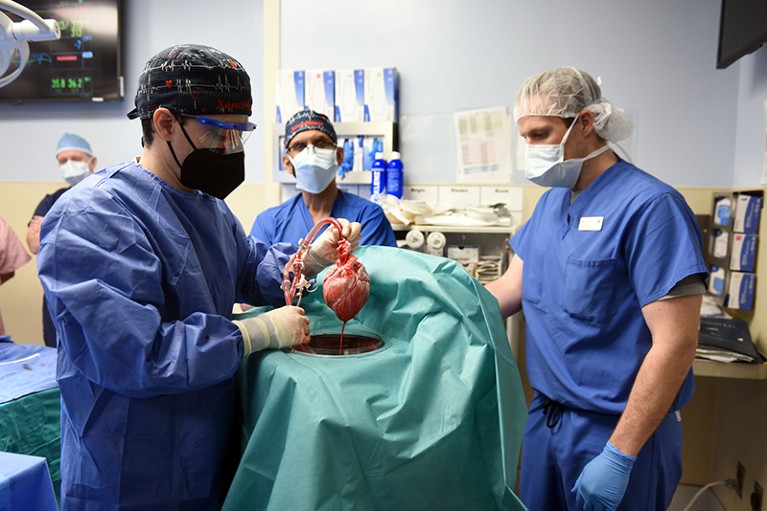
Members of David Bennett’s surgical team at the University of Maryland prepare the pig heart for transplant.Credit: University Of Maryland School Of Medicine/ZUMA
And although Fishman says it’s unlikely, viruses that infect the two species could recombine in the human body to create a new pathogen, much as influenza viruses do in birds, bats and pigs.
The FDA recommends that, to minimize public-health risks, pig organs be transplanted only into people who have no other option and whose quality of life would be significantly improved by the operation. The agency says it has policies for long-term patient monitoring and for prohibiting people with pig organs from donating blood because of the risk of disease transmission.
Bennett’s heart was found to harbour porcine cytomegalovirus (CMV), a member of the herpesvirus family that commonly infects pigs, but it’s still not clear whether the virus killed Bennett. Researcher Muhammad Mohiuddin at the University of Maryland Medical Center, who led the study that reported Bennett’s operation1, says that there is no evidence that the virus damaged the heart — and he is certain that it did not spread to the rest of Bennett’s body. He suspects that the patient’s organs had already been damaged by his illness, or by complications — independent of the pig virus — that occurred after the transplant.
Still, Mohiuddin can’t rule out that the virus played a part. Revivicor had tested its pigs for RNA from CMV, along with other pathogens, and had certified that they were clean. Mohiuddin and others suspect that the virus was latent in the organ and would have been detectable only by testing the animal’s antibodies. Revivicor says it has developed more sensitive CMV tests, but declined to say how many pathogens it screens for.
Mohiuddin, whose team received emergency permission from the FDA to conduct Bennett’s transplant, plans to perform more such operations once he is certain that future pigs are virus-free. He says that medical centres are constantly offering him patients who are good candidates and willing to receive a pig transplant, but he has turned them down. “Before we satisfy anyone else, I have to be satisfied myself,” he says.
Trial types
Although the heart xenotransplant attracted a lot of attention, the first solid organ to enter clinical trials is likely to be the kidney, says Tector, because it is simpler than many other organs.
For more complex organs, eGenesis is planning a transitional step. Within 12 to 18 months, it hopes to begin testing pig livers in a set-up that is similar to dialysis. Three to six participants close to death would each be temporarily hooked up to a separate pig liver; their blood would be routed through the organ to remove toxic waste products that build up during liver failure. Curtis says the company is also working on juvenile pig hearts that would similarly serve as a bridge for children with heart problems until they can get a human heart transplant.
At its June meeting, FDA officials laid out a list of concerns about xenotransplantation products, including the quality of organs and how to ensure they are free of pathogens.
“There’s a lot we have to answer,” says Tector. But, he says, 60–70% of Makana’s baboons live for more than a year with a functioning pig kidney, so the company feels ready to start clinical trials. It submitted an FDA application earlier this year.
When trials do begin, Fishman says, the most important thing will be to collect as much data as possible from each participant. “We owe it to the patient, and we owe it to society.”
