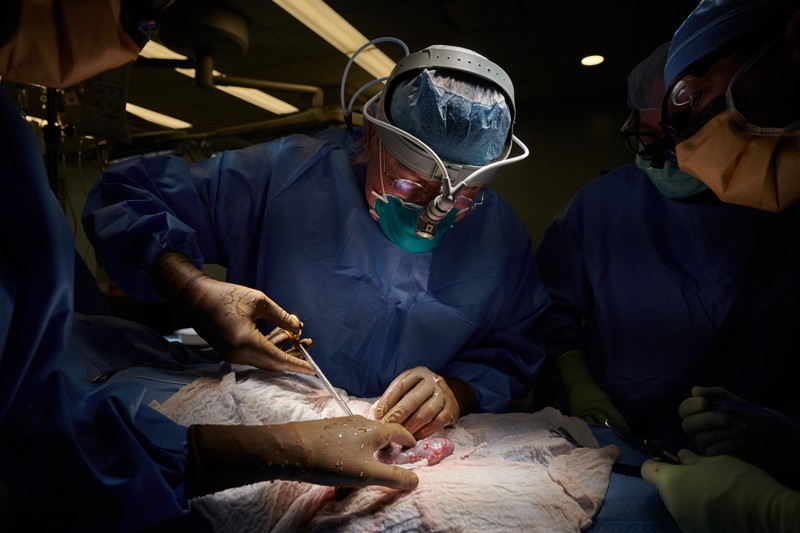
In the months since physicians showed that it is possible to transplant pig organs into humans, researchers have been calling for US regulators to allow clinical trials to test such procedures thoroughly in people. Last week, during a two-day meeting of an advisory committee to the US Food and Drug Administration (FDA), agency officials and physicians discussed what regulators would need to move forwards. Most attendees agreed that human trials are needed to help answer the most pressing research questions about inter-species transplants, known as xenotransplants.
The data support the initiation of “small, focused” clinical trials with “appropriately selected patients”, says Allan Kirk, a transplant surgeon at the Duke University School of Medicine in Durham, North Carolina, who presented at the meeting.
Researchers have repeatedly transplanted pig organs into non-human primates, such as baboons, with success. But these experiments don’t simulate human trials perfectly. If the ultimate goal is to do transplants in people, human trials are needed, says Caroline Zeiss, a veterinary specialist at Yale School of Medicine in New Haven, Connecticut.
Such trials, she says, would help to answer a slew of questions, including what is the best cocktail of immunosuppressive drugs to give humans to help their bodies accept a pig organ, and how can physicians manage the risk that transplanted organs might harbour a pig virus. Researchers also want to know which pig breed is best suited for growing transplant organs, and how co-occurring health conditions, such as diabetes, could affect transplantation success.
Physicians see an urgent need for the trials: more than 100,000 people are waiting for organ transplants in the United States alone. Researchers have long hoped that xenotransplantation could help to meet demand and, therefore, save lives. “We have people dying each day waiting for organs,” says Jay Fishman, a specialist in transplant infectious disease at Massachusetts General Hospital in Boston who participated in the FDA meeting.
A big question answered
Table of Contents
Although there have so far been no formal human xenotransplant trials, physicians have performed a handful of the procedures in the past year, with the permission of institutional ethics boards. In late 2021, for instance, surgeons transferred genetically modified pig kidneys into two legally dead people who had no discernible brain function and were on ventilators. The kidneys functioned normally over the 54 hours of the test and seemed to produce urine1.
In January this year, a severely ill man became the first to receive a pig heart, during an operation in Baltimore, Maryland. (The man otherwise faced certain death, so the FDA granted a compassionate-use authorization for the procedure.)
The heart recipient recovered from the surgery, and his body did not reject the genetically modified organ, but he died two months later. Physicians later found traces of porcine cytomegalovirus (PCMV) in the pig heart and now think that the pathogen might have contributed to the man’s death. An investigation is under way.
Fishman says it’s thought that the virus doesn’t infect human cells, but it has been linked with reduced survival times for non-human primates that received pig organs2. To get to the bottom of the mystery, more tests and trials are needed, researchers say.
Even though the heart-transplant recipient died, the surgery represents an enormous accomplishment, Kirk says. The science of xenotransplantation, he says, has advanced to the point that there is an answer to the biggest question — can a pig organ support life in a human who would otherwise die? And the answer is yes.
The high-profile transplants have “increased public awareness of the field” and have “made this an optimal time for public conversation” and clinical trials, said Wilson Bryan, director of the FDA’s Office of Tissues and Advanced Therapies in Silver Spring, Maryland, at the meeting.
Reducing risks
But there are still many questions that must be answered before xenotransplantation can become standard clinical practice. During the advisory meeting, the FDA sought advice from committee members on how to improve screening for viruses — PCMV can linger silently in infected pigs — and how to reduce the risk of breeding pigs with viral infections. To be more comfortable with human trials, Zeiss said she wants to see validated tests that could eliminate the possibility that PCMV and other viruses are lurking in pigs bred as organ donors.
Companies such as Revivicor in Blacksburg, Virginia, owned by United Therapeutics, have been breeding pigs for use in xenotransplantation. They have been searching for the right combination of genetic modifications for their pigs to help ensure that humans’ immune systems accept organs from the animals. These companies “have been creative making these pigs; hopefully they’ll be creative testing them”, said FDA investigator Deborah Hursh at the meeting.
Other panellists discussed whether it would be possible to develop a standard ‘package’ of immunosuppressive drugs for humans, and genetic modifications for pigs, to ensure success. Fishman said that there probably won’t be one package that works for everyone. Instead, it will need to be tailored on the basis of the organ being transplanted and the recipient’s condition, as is done in human-to-human organ transplants.
Researchers at the University of Alabama at Birmingham and the University of Maryland Medical Center in Baltimore have signalled their desire to begin xenotransplant clinical trials soon. The FDA hasn’t publicly indicated what it will do with the advice collected during the meeting, but a 30 June report from The Wall Street Journal says that the agency is devising plans to allow trials.