It is increasingly clear that, as humans age, many, if not most, of our tissues become composed of populations of cells, termed clones, that often harbour genetic mutations found in the malignant tumours that arise from the same type of tissue. Although these clonal populations seem to be normal cellular lineages apart from the cancer-associated mutations, little is known about how these cells respond to damage caused by exposure to ultraviolet light or toxic chemicals, for example. A process called metaplasia — the replacement of one cell type with another after tissue damage — increases the risk of cancer formation1. Why abnormal repair during metaplasia predisposes cells to form cancer is mainly unknown. Writing in Nature, Alonso-Curbelo et al.2 report a study of pancreatic cancer in mice that reveals how a mutation biases the outcome of metaplasia towards the development of cancer.
Most human pancreatic cancers contain mutations in the KRAS gene, which encodes a type of enzyme, termed a kinase, that has a key role in signalling. The KRAS enzyme contributes to the control of cell growth in healthy tissues, but cancer-promoting mutations cause enzyme hyperactivation that leads to continuous cellular growth. Epithelial cells in pancreatic ducts can, driven by cancer-promoting KRAS mutations, become a type of malignant tumour called pancreatic ductal adenocarcinoma (PDAC)3,4.
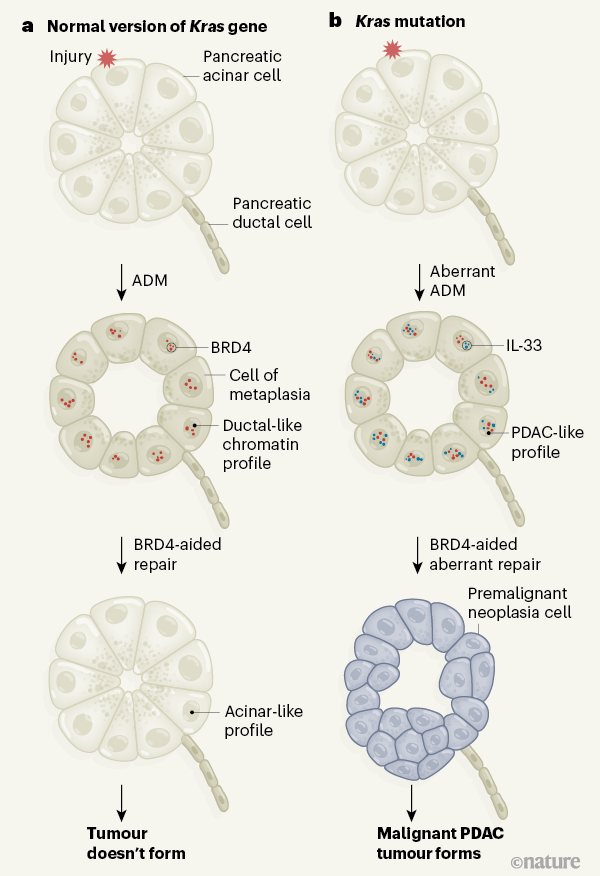
Generally speaking, KRAS mutations alone are insufficient to drive tumour development3,4; however, they can act in concert with environmentally mediated tissue injury to accelerate malignant transformation. This occurs by the aberrant regulation of metaplasia (Fig. 1), in which one type of pancreatic epithelial cell (an acinar cell) is reprogrammed temporarily into another sort (a cell similar to a ductal cell). This transition is called acinar-to-ductal metaplasia (ADM), and such an epithelial-cell-state conversion occurs in response to environmental stress1,5.
The authors sought to understand why mouse pancreatic cells with mutations in the Kras gene respond differently to environmental insult compared with cells lacking such mutations. Cells grown in vitro do not faithfully reproduce events inside living tissues, so Alonso-Curbelo et al. used sophisticated genetic engineering to develop mouse models. These animals enabled the authors to specifically track the fate of pancreatic acinar and ductal cells that had normal or mutant versions of Kras, and determine their response to tissue injury mediated by chemical treatment.
Consistent with previous work3,4, the authors found that the regeneration of damaged pancreatic cells after tissue-injury-mediated ADM was impaired in mice with a Kras mutation. Unlike normal mice, those with mutant Kras rapidly developed a type of premalignant cellular growth — described as pancreatic intraepithelial neoplasia — that is a precursor to PDAC formation3.
The rapid emergence of this neoplasia specifically in Kras mutant mice after tissue injury led the authors to speculate that aberrant regulation of chromatin (the complex of DNA and histone proteins in the nucleus) might explain how PDAC subsequently develops. To investigate this, Alonso-Curbelo and colleagues examined cells that had normal or mutant Kras to assess any genome-wide differences in chromatin accessibility. Accessibility here refers to genomic regions that are in an ‘open’ conformation that allows DNA-binding transcription-factor proteins to gain access to DNA and regulate the expression of nearby genes.
After injury, cells with Kras mutations gained a chromatin-accessibility profile that closely resembles that found in PDAC cells. By contrast, the newly accessible chromatin regions identified in cells with Kras mutations remained in a ‘closed’ conformation in normal pancreatic cells after injury. These data, consistent with findings6 published this year, suggest that the combination of Kras mutation and tissue damage by environmentally mediated injury drives an early step in the pathway to tumour formation by establishing a cancer-associated profile of chromatin accessibility before a complete malignant transformation of the cells occurs.
Sites of accessible chromatin often contain DNA sequences called enhancers that help to regulate gene expression. Alonso-Curbelo et al. reasoned that proteins involved in transcriptional regulation might act at regions of increased chromatin accessibility in cells with Kras mutations and drive the tissue’s damage-induced ADM to transition into pancreatic intraepithelial neoplasia. To investigate this possibility, the authors used a technique called RNA interference to decrease the production of BRD4, a transcriptional co-activator protein that is needed for the expression of many genes, particularly those whose expression is directed by clusters of enhancer elements termed super-enhancers7. This revealed that BRD4 is required both for tissue repair in normal cells following injury-induced ADM, and for ADM to lead to pancreatic intraepithelial neoplasia in the context of a Kras mutation.
When analysing genes potentially responsible for the development of pancreatic intraepithelial neoplasia, the authors focused on the gene Il33, whose expression increased swiftly on injury in cells with mutant Kras. Il33 encodes the protein interleukin-33, a type of inflammatory immune-system signalling molecule called a cytokine. Alonso-Curbelo et al. found that injured pancreatic cells with Kras mutations had greater chromatin accessibility in the region encoding Il33 and higher expression of Il33 compared with the situation in injured cells with normal Kras. Crucially, this increase in Il33 expression required normal expression of BRD4.
To confirm the importance of interleukin-33, the authors administered this protein to their tissue-injury model mice. The treatment accelerated the development of pancreatic intraepithelial neoplasia if the animals had a Kras mutation, but had no effect if the animals had normal Kras. Although the precise components of the transcriptional machinery needed for Il33 expression in a Kras-mutant context remain to be established, the authors clearly demonstrate that BRD4 has a role.
These data are consistent with the observation8 that first-generation drugs that non-selectively target gene-regulatory modules called BET bromodomains (BD1 and BD2), which are present in all BET proteins, including BRD4, have demonstrated some utility when tested in animal models or in the clinic against a broad range of cancers, including PDAC. Next-generation BET inhibitors can preferentially alter either the maintenance or the induction of gene expression by engaging, respectively, BD1 or BD2 of BET proteins9. Given that Il33 induction underpins the transition towards PDAC formation in cells with a Kras mutation, it would be interesting to test whether a BD2-selective inhibitor could forestall or curtail the transition to tumour formation.
In humans, pancreatic damage caused by environmental insults such as a high alcohol intake can result in an inflammatory condition called chronic pancreatitis, which markedly increases the risk of developing PDAC10. However, mechanisms that might explain this observation have remained elusive. This study by Alonso-Curbelo et al. highlights how the presence of cancer-associated mutations in what seem to be essentially normal cells can drive a striking alteration in how they respond to injury, thereby nudging them towards tumour formation by aiding the formation of a neoplasia.
Given that many of our tissues, including blood, skin and gut epithelial cells, have clonal cellular populations that are often subjected to environmentally mediated damage, Alonso-Curbelo and colleagues’ findings might have far-reaching implications. Their results should form the basis of future studies of how premalignant clones in other tissue contexts respond to injury. Such insights might, in turn, lead to drug targeting of the factors that drive abnormalities in tissue regeneration after the initiation of metaplasia.
Competing Financial Interests
Table of Contents
M.A.D. has been a member of advisory boards for GlaxoSmithKline, CTx CRC, Storm Therapeutics, Celgene and Cambridge Epigenetix. The Dawson lab receives research funding from CTx CRC.